Chapter 7: Precipitation Processes
Alison Nugent and David DeCou
Learning Objectives
By the end of this chapter, you should be able to:
- Recall the importance of cloud condensation nuclei and aerosols
- Calculate the speed of a falling cloud droplet and raindrop
- Describe the Collision-Coalescence process
- Describe the Ice Phase (Wegener-Bergeron-Findeisen) process

Introduction
Sometimes rain feels like a gentle mist but at other times its a heavy downpour that floods streets and sidewalks. Many times, clouds cover the skies but never produce any precipitation at all. This leads us to question: why does it rain and do raindrop sizes vary? What is the relationship between raindrops and cloud droplets, and by what processes do each form? You know that clouds form by condensation but, apparently, condensation by itself is a necessary but insufficient condition for rain. We will explore why this is by examining cloud droplets and raindrops in more detail.
The average cloud droplet is very small with an average diameter of about 20 micrometers (μm), which is the same as 20*10-6 m, 0.002 cm, or 0.02 mm. This diameter is about 100 times smaller than your average raindrop.
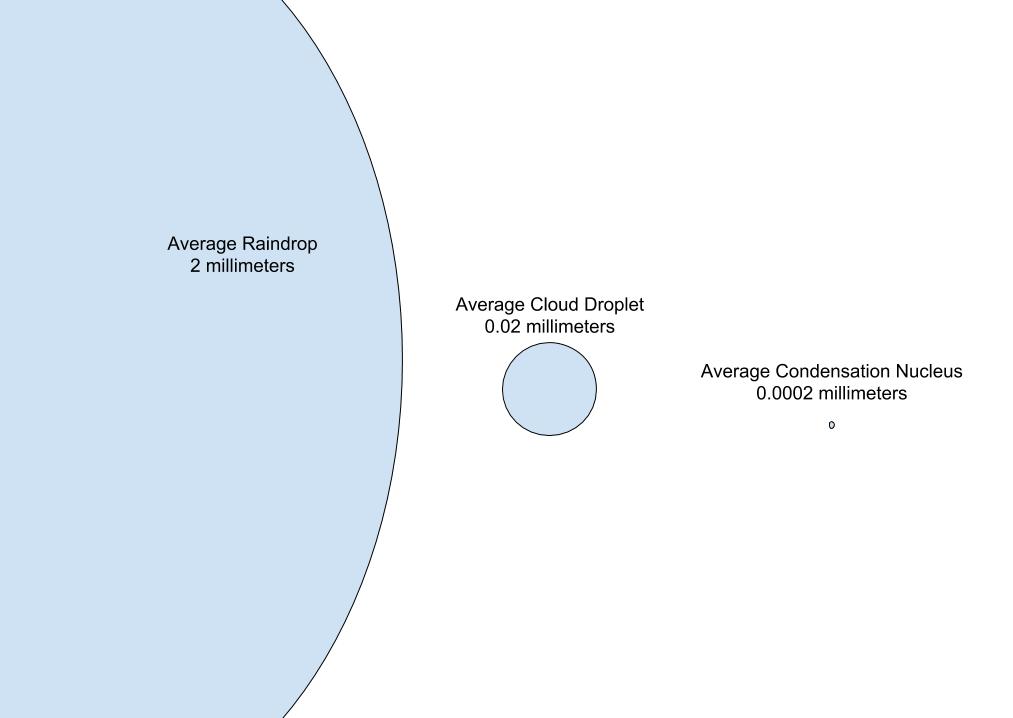
The following image gives a sense of the difference in scale between raindrops (left), cloud droplets (center), and cloud condensation nuclei (right). The average raindrop has a diameter of 2 mm, and the average condensation nucleus has a diameter around 0.0002 mm.
When considering the volume of the droplets or particles, this differences quickly grows. The following image shows the volume of various cloud droplets and rain drops on a log scale.

Notice how cloud droplet sizes range from 2 μm to 50 μm and raindrop sizes range from 200 μm to 2500 μm. Liquid drops exist on a size spectrum from about 1 μm to almost 5,000 μm (or 0.5 cm). The minimum size for a cloud droplet is effectively set by the surface tension required to keep the H2O molecules together. The smaller the droplet, the higher the surface tension necessary. The maximum size for a raindrop is limited by drop breakup because when the drop becomes too large, air friction will break it up into a bunch of smaller droplets.
In general, the only difference between a cloud droplet and a raindrop is that a raindrop has a non-negligible fall velocity. On a continuous spectrum of sizes, at some point the gravitational pull on water drops in the atmosphere becomes large enough not to ignore. While all drops will fall, the larger the drops are, the faster they fall.
Cloud Droplets
Recall from the previous chapter on clouds that cloud condensation nuclei (CCNs) are required for water vapor to condense onto. Many CCNs are hygroscopic, meaning they tend to absorb moisture, so condensation may start before the relative humidity reaches 100%. For example, when condensation occurs on salt particles, which are extremely hygroscopic, condensation can begin at 80% relative humidity or lower.
Imagine there are many CCNs of differing sizes in a body of humid, but unsaturated air. If the air were to be lifted by a mountain or in a rising thermal, it would cool, and the relative humidity would increase. As the air nears saturation, condensation will begin to occur on the largest and most hygroscopic CCN. At some later time, a cloud of many small cloud droplets, far too small to fall at any significant speed, will form.
The terminal velocity of a falling cloud droplet (with radius “r” less than 40 μm) is given by the following equation from Stoke’s Drag Law:
![]()
where “r” needs to be expressed in meters. A simple calculation will show that it takes hours, if not days, for a cloud droplet to fall from even low altitudes. The friction provided by the air or even tiny upward air currents will keep cloud droplets suspended for long periods.
Terminal velocity implies that a steady state has been reached in the fall velocity with a balance between the downward gravitational force on the drops and the upward frictional drag of air.
Raindrops
For raindrops, a different equation is used to approximate the fall speed. For spherical raindrops,
![]()
where ρ0 is a reference value of density, typically 1.2 kg m-3 and ρair is the density of air where the raindrop exists. Again, “r” is the radius of the drop, given in meters. High up in the atmosphere when ρair is small, the speed of a falling raindrop, wT, rain, will be faster than near the surface when ρair is similar magnitude to ρ0. As the air density increases, the frictional drag on a drop also increases.
Note that this equation for raindrops is a vast simplification because raindrops are not typically spherical shaped. As they fall, the passing air deforms them into pancake shaped drops. However, this equation provides an approximation for fall velocity.
So how can cloud droplets grow to form raindrops? The condensation process is not enough and is far too slow to produce raindrops. From a volumetric view, it takes 1 million cloud droplets (10 μm radius) to combine together to make one single raindrop (1000 μm = 1 mm radius). There has to be another faster process by which cloud droplets can grow or combine together to become large and heavy enough to fall.
We will discuss two primary rain formation theories.
- Collision-Coalescence Process
- Ice Phase Processes (Wegener-Bergeron-Findeisen)
Collision-Coalescence Process
In warm clouds, where all of the cloud droplets are liquid, the collision-coalescence process is the primary mechanism responsible for producing precipitation. This is thought to be the case especially over tropical oceans. The collision-coalescence process is exactly as it sounds: cloud droplets collide and coalesce or stick together. Larger cloud droplets have slightly higher terminal velocities, because they have a smaller surface-area-to-weight ratios. This advantage allows them to fall faster and collide with smaller cloud droplets. Sometimes the cloud droplets will stick together and coalesce to form a larger droplet. This begins a positive feedback where these larger droplets then fall even faster, collide with even more smaller droplets in their path, and aggregate more and more cloud droplets together. However, note that collision between cloud droplets does not always mean that coalescence will occur. Sometimes droplets will bounce apart during collision if their surface tensions are too strong. For collision-coalescence to begin, a cloud needs to have a wide distribution of cloud droplet sizes. This can occur from a variation in CCN type—for example, sea salt aerosols are particularly large—or from random collisions between droplets.
The total amount of liquid water in a cloud as well as the time that a cloud droplet spends inside of a cloud influences how large it can grow through the collision-coalescence process. The cloud height is of course a factor here, but its a little more complicated than that. Rising motion in a cloud will slow the downward speed of a falling droplet. This can act to increase the amount of a time a cloud droplet spends in a cloud, as well as the size it will grow. Let’s cover a few examples.
Deep cumulus clouds with convective updrafts tend to produce larger raindrops because upward motion is strong and droplets have a long time in the cloud to grow. In fact, the droplets need to become sufficiently large in order for their fall velocity to overcome the updraft velocity.
On the other hand, stratus clouds are typically not very thick and have weak updrafts, so droplets in these clouds don’t spend a long time in the cloud itself, and therefore are not be able to grow very large. If there is moist air below the stratus cloud, the drops may reach the ground as a light drizzle. However, if there is dry air below the stratus cloud, the drops may evaporate before they are able to reach the ground.
To summarize, in warm clouds, cloud droplets grow to precipitation sized drops through the collision-coalescence process. The most important factor in raindrop production is the liquid water content of a cloud. Assuming the cloud has sufficient water, other factors that affect raindrop production are: thickness of the cloud; strength of updrafts within the cloud; cloud drop distribution of sizes; and difference in electric charge of the droplets and the cloud itself. Thin stratus clouds with weak vertical motion may produce weak drizzle, if any, while tall cumulus clouds with strong updrafts can produce heavy rain showers. The following image illustrates the collision-coalescence process of raindrop production.

Ice Phase Process

Outside of the tropics, the ice phase process of rain formation is the primary mechanism producing most of the worlds precipitation. The ice phase process occurs in cold clouds or clouds with temperatures below 0°C. To understand why, we need to know something about freezing of liquid water droplets.
Supercooled Water and Ice Nuclei
Supercooled water is liquid water that exists below the freezing point of water (below 0°C). Similar to how cloud droplets need a surface on which to condense, ice crystals also need a nucleus or ice embryo to freeze. Without an ice nucleus, liquid water drops can remain liquid in temperatures as low as -40°C. Once beyond -40°C, all hydrometeors (water particles) will exist in the solid state. Typically the distribution of liquid and solid hydrometeors in a cloud looks like the following image.

At low elevations above freezing (region 4), the hydrometeors in the cloud exist as liquid droplets. Above the freezing level (region 3), supercooled liquid droplets exist. Above that, some liquid droplets begin to freeze, and both liquid and ice phase hydrometeors co-exist (region 2). Finally, above some level where the temperature is cold enough, all hydrometeors will exist in their solid state (region 1).
When liquid water droplets freeze without any sort of nucleus, this is known as homogenous or spontaneous freezing. While this occurs within a large body of freshwater at temperatures slightly below 0°C, cloud droplets will not freeze spontaneously until temperatures are -40°C or lower.
For droplets to freeze spontaneously, enough molecules within the droplet must form a rigid pattern and become a tiny ice structure known as an ice embryo. When this embryo grows sufficiently large, at a certain size it will act as an ice nucleus, which are described below. The other molecules in the droplet then become attached to the ice structure and the entire droplet freezes.
Tiny ice embryos are able to form when water drops just below freezing, but typically at these temperatures there is enough thermal agitation to weaken their structure and break them apart. At lower temperatures, there is less thermal motion, and ice embryos have a better chance of growing large enough to freeze the surrounding water. When you have larger volumes of water, ice embryos have a better chance of growing large enough to freeze the surrounding liquid before being broken up, but this becomes more and more difficult with smaller volumes of water. Only the largest cloud droplets can freeze spontaneously without a nucleus at temperatures below -40°C. In most cases, ice nuclei are required for ice crystals to form in sub-freezing clouds.
Just as CCNs are required for liquid cloud droplets to form, ice crystals form on particles called ice nuclei (IN). Particles serve as effective IN if they have similar geometry to an ice crystal, for example, ice itself is an effective IN. There are not many IN in the atmosphere, especially at temperatures above -10°C, but certain types of particles become active IN with lower temperatures. For example, dust can be an effective IN. Ice nuclei are rare compared to hygroscopic cloud condensation nuclei.
Some IN allow water vapor to immediately become solid ice when they come in contact together. These are known as deposition nuclei because the water vapor changes phase directly into solid ice without becoming liquid first (phase change from gas to solid is called deposition). IN that are effective at causing the freezing of supercooled liquid droplets are called freezing nuclei. Some freezing nuclei must be immersed in a liquid drop in order to freeze it, while others are effective at inducing condensation and then freezing. Many freezing nuclei will cause supercooled droplets to freeze as soon as they collide, which is called contact freezing, and these nuclei are referred to as contact nuclei. These different freezing methods are outlined in the figure below.

To summarize, cloud droplets may freeze spontaneously, but only at very low temperatures. Ice nuclei can help the growth of ice crystals, but they are not naturally abundant.
Saturation Vapor Pressure
So, we have cold clouds that contain many more liquid cloud droplets than ice crystals, even at sub-freezing temperatures, and these particles are not large/heavy enough to precipitate out of the cloud. How do we get rain and snow out of the ice-crystal process then?
Imagine a cloud with super cooled liquid water and saturated air. At saturation, the liquid droplets are in equilibrium with the water vapor in the air. The number of water molecules leaving and entering the surface of the liquid droplets are equivalent. Now imagine that an ice crystal forms by one of the processes described above. In the below-freezing portion of a cloud, this ice crystal is surrounded by many liquid supercooled droplets. Because the saturation vapor pressures with respect to liquid and ice are slightly different, the presence of this new ice crystal has a big impact on the cloud.

With respect to liquid, the liquid droplets were at saturation. But now, with respect to ice, the ice crystal is in an environment that is supersaturated. You can think of this as the following: it is easier for water molecules to escape a liquid surface through evaporation than to escape a solid surface. This means that there will be many more molecules escaping the liquid water surface at a given temperature and will require more water vapor around it in order to keep the droplet in saturation. At the same temperature, the saturation vapor pressure above a surface of water is greater than the saturation vapor pressure above a surface of ice.
This difference in saturation vapor pressure with respect to water and ice causes water vapor molecules to deposit from the environment onto the ice crystal. Because the vapor molecules are being removed from the environment around the liquid droplet, the vapor pressure with respect to the water surface decreases. This throws the water droplets out of equilibrium with their surroundings, causing them to evaporate, replenishing the removed water vapor from the environment. This provides additional moisture for the ice crystal, allowing it to grow at the expense of the liquid droplets.
This process is called the Wegener-Bergeron-Findeisen (or more generally, the ice phase) process. Ice crystals in a sub-freezing region of a cloud will grow larger at the expense of surrounding water droplets.
Falling Ice Crystals
Imagine this happening throughout a large cloud. The water vapor within the cloud as well as the water vapor from evaporating supercooled liquid droplets provides a continuous source of moisture for ice crystals, allowing them to grow rapidly. Eventually, these ice crystals become large enough to fall. The same issues exist with updrafts and rising air, but at some point the crystals will fall faster than the updrafts within the cloud.
Sometimes, ice crystals collide with nearby supercooled droplets in the cloud, causing them to freeze onto the crystal as ice. The ice crystal will grow larger and larger as it collides into more droplets, this is called riming or accretion. This forms an icy clump called graupel (also known as snow pellets), which may break apart into tiny ice particles as it collides with more droplets. These splinters may form graupel of their own as they collide into other droplets, which in turn may also splinter, causing a chain reaction.
In clouds that are colder, ice crystals may collide together and break apart into smaller ice particles, which act as tiny seeds that can freeze supercooled droplets on contact. This could also cause a chain reaction that produces many ice crystals. As these ice crystals fall, they can collide and stick together. This process of collision and sticking is called aggregation. A fully grown ice crystal is what we call a snowflake.

There must be many times more water droplets in a cloud than ice crystals for ice crystals to get large enough to fall as snow—on the order of 100,000:1 to 1,000,000:1.
Precipitation Types
The ice phase process accounts for most global precipitation. The term precipitation refers to all types of precipitation—from fog, rain, snow, hail, etc. Anything composed of water that is falling in the atmosphere can be called precipitation. However, we know that not everywhere in the world gets snow all the time. While the ice phase process helps with precipitation formation, many things can happen to falling drops along their journey from the cloud to the ground. Here are a few examples.
Rain: Ice crystals melt before they hit the ground.
Snow: Ice crystals collide and stick, forming a fully grown ice crystal, and falling to the ground as a snowflake.
Graupel: Ice crystals collide and stick with other ice crystals forming clumps of snow called graupel.
Sleet: A mixture of rain and snow, formed by partial melting.
Freezing Rain: Supercooled liquid rain that freezes on impact with the surface.
Ice Pellets: Ice crystals melt before they hit the ground, refreeze in a cold layer, usually just above the surface, and end up falling as frozen rain drops.
Hail: Ice crystals that repeatedly pass through a supercooled liquid region of cloud where riming builds up on the hydrometeor. The formation of hail requires strong updrafts and a relatively long time inside of a cloud.
As you can see, the journey of an ice crystal after formation is not always straightforward and depends strongly on the environmental conditions, especially temperature. In the following chapters (especially chapter 12), we will learn how airmasses and fronts combine together regularly in Earth’s atmosphere to create conditions that are conducive to all types of precipitation.

The above image provides an example of how a warm front creates temperature gradients in the atmosphere that produces precipitation types from rain, freezing rain, sleet, and snow depending on your location with respect to the front.
Chapter 7: Questions to Consider
- Which rain formation process produces most of the worlds precipitation? What type of cloud does this process occur in?
- What factors determine how large a cloud droplet can grow through collision-coalescence?
- Match the ice nucleation methods:
-
Selected Practice Question Answers: