Chapter 3: Thermodynamics
Alison Nugent
Learning Objectives
By the end of this chapter, you should be able to:
- Define and describe four methods of energy transfer
- Describe the change in energy associated with changes in water state
- Define and apply the first law of thermodynamics
- Differentiate Eulerian and Lagrangian frameworks
- Describe the importance of the dry adiabatic lapse rate, and recall what sets its constant value in the atmosphere
- Compute potential temperature and apply the conserved variable approach
- Draw a diagram of surface heat fluxes and Earth’s radiation budget
- Compute the Bowen ratio, and define latent and sensible heat flux
Introduction
What is Energy? You may hear frequently about “green energy”, “clean energy”, “renewable energy”, and “solar energy” in the media, as energy is currently a hot topic. Power plants, wind turbines, and solar panels may come to mind. The first paragraph in Roland Stull’s Practical Meteorology Chapter 3 discusses several types of energy right off the bat, but first of all, what is energy?

Your intuition will probably tell you that we need energy in order to do things. Energy makes stuff “go”. Your appliances, your car, and your body all need energy. Your body utilizes energy to maintain its various functions even as you read this sentence. You know that energy is a real thing that exists; you see evidence of it everywhere. The heat from your stovetop, the ice melting in your glass, and that thunderstorm rolling in are all evidence of energy at work, on scales both micro and macro.
Energy can be defined as the ability to do work. When you apply a force on an object, it is said that work is done on the object if that object is displaced, meaning it moves from its original location. For example, when you pick up a book, you exert a force against gravity causing the book to change position, and you do work on the book. The higher you lift the book or the further you throw it (should you decide to), the more work you do. However, if you apply a lot of force on a heavy piece of furniture but it doesn’t move from its original place, no work has been done regardless of the amount of effort.
![]()
![]()
Internal energy is the total amount of energy stored in any object and determines how much work the object is capable of performing. This includes both kinetic energy (energy that an object has when it is in motion) and potential energy (energy that is stored). For example, a bowling ball sitting on a table contains energy despite the fact it is not in motion. It does not have kinetic energy because it is still, but it contains potential energy simply because of where it is situated. Were it nudged off the table, the bowling ball will do work because it will be pulled downward by gravity. This is an example of gravitational potential energy. The potential energy (PE) due to gravitational pull is given by the following equation:
![]()
![]()

Anything that moves contains kinetic energy (KE), which is given by the following equation
![]()
where m is the mass of an object in kilograms (kg) and v is the velocity of an object in meters per second (m·s-1). From this relationship you can see that objects with more mass or objects that are moving faster have more energy.
Energy takes on many forms and often changes forms from one to the other, but the total amount of energy in the universe remains constant. Energy cannot be created or destroyed. This means that the energy lost in a process must be the same as the energy gained in another. This is what the law of conservation of energy means, and this is what is known as the first law of thermodynamics. The first law of thermodynamics frequently comes into play in atmospheric motions and will be discussed further later in this chapter.
In short, energy is the capacity of a system to perform work. Energy is always conserved and cannot be created or destroyed. We begin this chapter with a short review of energy because energy is ultimately responsible for Earth’s weather from temperature changes in the atmosphere to the resulting air motions. Without energy, no weather would occur.
Energy Transfer
One important example of kinetic energy is thermal energy, which comes from the tiny movement of many molecules in a system. In Chapter 1, we discussed how temperature is a measure of the average speed of atoms and molecules in a system. Here we can further describe temperature as being proportional to the average kinetic energy of the random motions of the molecules in a system. The faster the molecules move, the higher the temperature.
The transfer of thermal energy due to the temperature difference between two objects is what is known as heat. Heat is a form of energy in transit, and once transferred, it is stored as internal energy. There are four main methods of heat transfer: conduction, convection, radiation, and the absorption or release of latent heat.

When you directly touch a hot object, such as the stovetop, the energy from the hot stove top is immediately transferred to your cool hand due to a difference in the speed of the molecules, causing you to feel a burn. This is an example of conduction: energy directly transferred through a substance without the movement of material. Certain materials are better conductors of heat than others. Metal, for example, conducts heat very efficiently, while air, which acts as an insulator, is a very poor conductor of heat.
Another type of energy transfer is convection, which occurs in liquids and gases (both fluids) because molecules can move freely and currents can naturally occur in them. This happens constantly in the atmosphere. Convection refers to movement within a fluid due to the tendency of lower density fluid to rise over higher density fluid, which sinks due to the force of gravity resulting in heat transfer within the fluid.
In the figure below, a beaker is being heated from the bottom by a flame. The arrows show upward movement in the center where the fluid is heated and therefore is less dense and buoyant. The cooler fluid at the top of the beaker is more dense and sinks toward the bottom under the influence of gravity.

Finally, when it comes to the atmosphere, the most important form of energy is the energy we get from the Sun, which is called radiant energy or radiation. Radiation is another type of heat transfer that was covered in Chapter 2. Remember that radiant energy can be transferred to an object without the space in between being heated. This is the result of electromagnetic waves from the Sun, which are then absorbed by the Earth’s surface or atmosphere and converted to thermal energy. Electromagnetic waves do not need any matter to travel through and propagate at the speed of light in a vacuum which is about 300,000 km·s–1.
Heat, or the transfer of thermal energy due to the difference in temperature between two objects, is defined as ∆q and is given in standard units of joules per kilogram (J·kg–1). One joule (J) is the standard unit of energy or work (kg·m2·s-2).
Latent Heat
Latent heat refers to the amount of heat that is added or removed from a system when a change of phase takes place. In atmospheric science, this almost always refers to the phase changes of water (H2O).
When heat is added to an ice cube, the temperature of the ice will increase until it reaches its melting point (0°C). After this point, any further addition of heat will cause the ice to melt to liquid water, but the temperature of the ice-water system will not change until the phase change is complete. The heat that is absorbed by the ice-water system from the environment in order for the phase change to occur is known as latent heat.
You have experienced this as your ice melts in your water glass. It stays cold until all of the ice is gone and then rapidly warms. The absorption of latent heat is also the reason you feel cold right after leaving your shower or a body of water. The excess water on your skin starts to evaporate, but it requires additional energy in order to transition from liquid to vapor. Energy is absorbed from the environment (your skin) to evaporate the remaining liquid, causing your skin to cool. This is known as evaporative cooling.
Processes that take heat from the environment (melting, evaporation, sublimation) are considered cooling processes. On the other hand, processes that add heat to the environment (condensation, freezing, deposition) are warming processes because during the phase change energy is released. This is an important distinction, so be sure this is clear before moving on. I’ll repeat it below for emphasis.

The reason phase changes of water are so important in the atmosphere is because it acts as the energy to fuel convection and thunderstorms. When water vapor condenses to form the liquid water found in clouds, this process releases enormous amounts of latent heat to the environment.
Latent heat (QE) is the latent energy that is possessed by an object of mass m, but usually we want to know the change of latent heat that is caused by a phase change process.
![]()
The change in latent heat is equal to the latent heat factor multiplied by the mass of water undergoing a phase change.
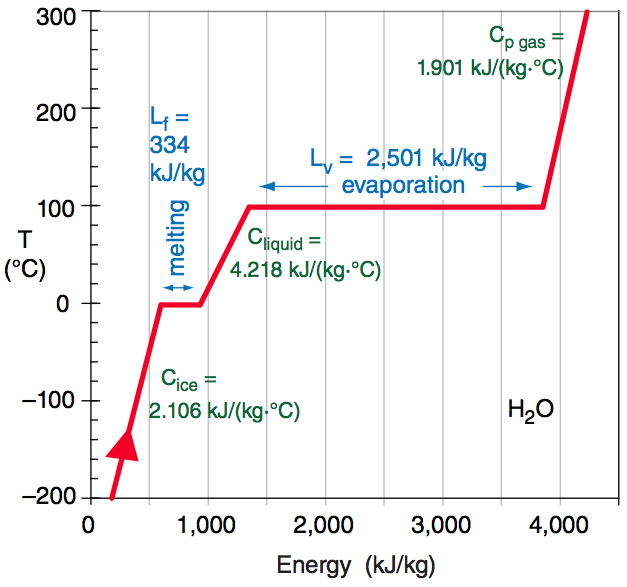
Different phases of water have different latent heat factors (L), meaning the change in phase of a mass of water corresponding to the change in latent heat is different depending on what process is taking place. This is well displayed in the image above by the horizontally flat regions where energy is going into the system but no temperature change is occurring.
For evaporation or condensation (phases changes between liquid water and vapor), the latent heat of vaporization, Lv = 2.5 * 106 J·kg–1 is used. For melting or freezing (phase changes between ice and liquid water), the latent heat of fusion or freezing, Lf = 3.34 * 105 J·kg–1 is used.
Specific Heat
Latent heat is the term for the amount of heat released from changes in phase, but what about changes in temperature unassociated with phase changes? These portions are the slanted lines in the figure above where energy is going into the system and the temperature is responding. Specific heat or heat capacity (C) is the term for the amount of heat required to raise the temperature of a substance by a certain amount. Typically this is defined at a constant pressure (Cp) or a constant volume (Cv).
Some substances, such as water, require a particularly large amount of heat energy in order for the temperature to change. The heat capacity of a substance is the ratio of heat energy that is absorbed to the corresponding rise in temperature. The specific heat is the heat capacity of a substance per a unit mass. Basically, specific heat is the amount of heat that is needed in order to raise the temperature of one gram (g) of a substance by one degree Celsius, or Kelvin.
For example, the specific heat of pure water is 4186 Joules per degree Kelvin per kilogram (J·K-1·kg-1), while the specific heat of dry air at sea level (Cpd) is about 1004 J·K-1·kg-1. The specific heat of dry air at constant volume (Cvd) is equal to 717 J·kg–1·K-1. This means that it takes much more energy to raise 1 kg of water by 1°C than it does for 1 kg of dry air. Remember that Celsius and Kelvin have a linear relationship so the change in temperature of one degree Celsius is the same as a change of one degree Kelvin (K = °C + 273).
Hawaii Focus Box
The difference in specific heat capacity of water and land leads to different annual cycles of air temperature and water temperature in Hawaii. The heat capacity of sea water is about 3985 J·K-1·kg-1 while the heat capacity of land is typically less than 1000 J·K-1·kg-1. This means that land heats up faster and cools down faster while the ocean takes longer to warm and also takes longer to cool.
The warmest temperatures in Hawaii typically follow the solar cycle with a slight lag. The warmest temperatures in the Northern Hemisphere occur during the time periods where the Sun’s angle is highest and the length of day is longest as we discussed in Chapter 2. This typically corresponds to June through August. However, the warmest ocean temperatures lag the land temperatures significantly by a few months because water takes more energy to warm. The warmest ocean temperatures occur from August through October.
There is some feedback where the warm ocean temperatures affect land temperatures as well, but it all comes down to the difference in specific heat.
First Law of Thermodynamics
As stated previously, the first law of thermodynamics states that energy is neither created nor destroyed. The total amount of energy must be conserved. In the atmosphere, this means that the amount of heat applied to a mass of air (thermal energy input) must equal the total sum of the warming of the air, plus the amount of work done per unit mass of air.
When heat (∆q, J·kg–1) is added to a mass of air (often referred to as an air parcel, see below), some of the added thermal energy warms the air, which increases its internal energy. When the air parcel warms, one of two things must happen. Recall the ideal gas law from Chapter 1: P=ρ*Rd*T. If temperature increases, (1) density must decrease to keep pressure constant or (2) the pressure will increase. Small pressure differences in the atmosphere always equalize first, so as the air warms, the density of the air parcel decreases. The decreased density of the air parcel gives it a lower density than the surrounding air, and it is therefore buoyant and begins to rise. As the parcel rises, it expands in volume in order to maintain equilibrium with the lower pressure outside the air mass, and it pushes into the surrounding atmosphere. Because of this, some of the thermal energy that is added to the air goes into doing the work of expansion and not all of it is used for warming.
![]()
![]()
For the atmosphere, a more usable form of the First Law of Thermodynamics is:
![]()
![]()
In the atmospheric form of the First Law of Thermodynamics, Cp*ΔT can also be redefined as Enthalpy (h), a term to quantify the total heat content of an air parcel.
![]()
![]()
![]()
This gives us the first term in the above equation for the first law of thermodynamics.
Frameworks for Understanding the Atmosphere
All atmospheric science concepts are shared with other disciplines like physics and chemistry, but they often use a more specific set of equations or variables as shown above with the first law of thermodynamics. This helps to simplify things because general physics equations can be applied to nearly any form of matter, while in atmospheric science we primarily deal with gases that are not constrained to a specific volume.
In the two sections that follow, two important frameworks are described that will help you to view the atmosphere in ways that will simplify concepts in the sections and chapters to come.
Lagrangian vs. Eulerian
When we look at or try to solve a problem in the atmosphere, there are two different lenses or frameworks through which we can view the problem. An Eulerian framework is a fixed framework, relative to a single point on the Earth’s surface. When a weather forecast is done for a given location on Earth or when you look at a dataset from one weather station, you are viewing the atmosphere from an Eulerian perspective — that is, how the wind and air travels past a fixed point. With a Eulerian framework, we need to be concerned about things like temperature and moisture advection, properties that travel with and are carried by the wind.
Another framework is Lagrangian, which is a framework that is constantly moving and travels with the air. When we are looking at motions within the atmosphere, such as rising or sinking air, it is useful to use this framework as a way to see how properties within the rising plume of air are changing. Both frameworks will be used in the coming sections and chapters.
Air Parcels
At this point, we should discuss a naming convention used in atmospheric sciences. Many times it is useful to think about a mass of air instead of individual molecules. We generally call a mass of air an “air parcel”. This is especially useful for distinguishing processes happening within the air, versus processes happening within the environment. An air parcel is often thought of as an amorphous bubble or blob of air, roughly the scale of a party balloon or a hot air balloon that contains uniform properties (temperature, density, pressure) throughout. Air parcels are simplified theoretical constructions used as a way to think about and examine motions and instability in the atmosphere.
For example, let’s revisit the important topic of latent heating. We discussed how condensation of water vapor to liquid water is a warming process. This is only clear if we separate the processes happening within a parcel of air and within the rest of the environment. When water vapor condenses within an air parcel, it gives off energy, which acts to warm the environment. We will often refer to air parcels when we want to distinguish changes within a piece of air with respect to the rest of the atmosphere.
In reality, as air parcels move through the atmosphere, there will be some mixing between the air parcel and the environment, and heat can be exchanged with the environment or added via radiation. However, with the concept of air parcels, we simplify the situation and imagine that radiative effects are small, and that mixing only occurs on the outer edges of the parcel, with a protected inner core.
Defining Changes in the Atmosphere
In order to study changes in temperature, momentum, and moisture in an air parcel, a Lagrangian framework is always used. With a Lagrangian framework, we can see changes from the parcel’s perspective as it moves, not from a fixed point on the ground.
Applying the hydrostatic equation from Chapter 1 to the first law of thermodynamics, we can get the Lagrangian first law of thermodynamics equation for a moving air parcel.
![]()
∆q, or heat transfer, can be caused by various processes, outlined in the following figure. The figure shows an air parcel moving both horizontally (through advection) and vertically (through convection), as well as the various processes both inside and outside that are causing heat exchange. The temperature inside the air parcel is conserved unless heat is transferred to or from the environment, or if it loses or gains heat by rising or sinking, which will cause it to expand or contract, respectively.

Lapse Rates
The atmospheric lapse rate, Γ, denoted by an upper-case gamma, is defined as the change in temperature with altitude, specifically a reduction in temperature with altitude.
![]()
A positive lapse rate indicates that the temperature is decreasing with increasing height, while a negative lapse rate indicates a “temperature inversion” meaning that the temperature is increasing with increasing height. Lapse rates are typically defined for:
- The environment, Γe
- A dry air parcel, Γd
- A saturated air parcel (moist), Γm
We can observe the environmental lapse rate by using atmospheric sensors attached to weather balloons. As a weather balloon rises through the atmosphere, it measures temperature and other properties on the way. The environmental lapse rate varies depending on time of day, altitude, latitude, land surface properties, heat fluxes, and air movement. A typical Γe is around 6.5 K km-1 but this will be discussed in later chapters. You will have experienced this decrease in temperature with altitude if you have hiked in the mountains or seen an outdoor thermometer reading on a commercial flight.
Dry Adiabatic Lapse Rate
In atmospheric science, you will often hear the word “adiabatic”, typically accompanied by the words “cooling”, “warming”, or “lapse rate”. An Adiabatic process just means that there is no heat transfer taking place (∆q = 0) during the process. For an air parcel, this means that no thermal energy is entering or leaving the air parcel from the outside. However, internal processes are allowed, such as the ones shown in the figure above (most notably adiabatic expansion and latent heating). An adiabatic lapse rate indicates that air is cooling or warming with altitude without any external heat exchange. An air parcel that contains no liquid water or ice (none of the moisture in the parcel has condensed into liquid, no saturation or latent heat release), will cool at the dry adiabatic lapse rate.
![]()
The reason that an air parcel expands adiabatically as it rises is due to the fact that the environmental air pressure decreases with height. The air parcel’s pressure will adjust to the lower pressure of its environment, but the parcel must expand in order to do so (in order for the air molecules inside the parcel to exert a smaller force). The parcel uses some of its own internal energy to do the work of expansion, and its temperature decreases as a result. The opposite is true as an air parcel sinks, the environmental pressure rises, and the parcel’s pressure adjusts in order to maintain pressure equilibrium, and the parcel must shrink for its pressure to increase. As it shrinks, work is done on it, and the temperature rises.
In later chapters we’ll define the moist or wet adiabatic lapse rate (Chapter 4), as well as the environmental lapse rate (Chapter 5) and their significance.
Potential Temperature
The potential temperature, θ, of a parcel completely ignores the temperature change of the parcel due to it having done work or been worked on (expanding or contracting). As a result, potential temperature is constant for an adiabatic process and θ does not change when ∆q = 0. Potential temperature is proportional to the sensible heat contained in a parcel and can increase or decrease when sensible heat is added or removed through diabatic (non-adiabatic) processes (∆q ≠ 0). Examples of diabatic heating include turbulent mixing, condensation (latent heat), and radiative heating. Potential temperature has units of K or °C, and can be found if you know the air temperature, T, at pressure-level, P, using the following equation
![]()
where Rd/Cp is a constant equal to 0.28571, and has no units, and P0 is a reference pressure, typically 1,000 hPa (100,000 Pa), or the local surface pressure.
Introduction to Thermodynamic Diagrams
Potential temperature is also very useful in thermodynamic diagrams, which will be briefly introduced here, but covered in more detail in Chapter 5. Thermodynamic diagrams are useful in diagnosing the state of the atmosphere and the buoyancy of air parcels by comparing the temperature difference ∆T between the parcel and its environment. Parcels that are warmer than their environment will tend to rise due to lower density, and the change of the parcel’s temperature with height can be anticipated based on the parcel’s moisture content. In the thermodynamic diagrams you will use, dry adiabat lines will be plotted to show the dry adiabatic lapse rate, as parcels will cool at this rate until condensation occurs within the parcel. Dry adiabats are labelled with θ because θ is constant along these lines. A simple example is provided here.

Heat Budget at Earth’s Surface
Before moving on from thermodynamics, let’s add another layer of complication to our understanding of Earth’s surface heat budget. In Chapter 2 we discussed how the Earth’s heat budget could be defined by the incoming shortwave radiation (K↓), reflected shortwave radiation (K↑), longwave radiation emitted by the Earth (I↑), and the downwelling longwave radiation emitted from the atmosphere (I↓) received by the Earth’s surface.
![]()
Earth’s surface is considered to be infinitesimally thin with no volume, and no heat can be stored, so the sum of all incoming and outgoing heat fluxes at the surface must balance. The net heat flux at the surface must be zero. In addition to the incoming and outgoing shortwave and longwave radiation, there are three other fluxes that must be considered. But first, let’s discuss what is meant by a “flux” of heat.
Heat Flux
Let’s say you have a cube of air, somewhere fixed relative to the ground. From an Eulerian framework (fixed-location), pressure changes can be neglected in the First Law equation, as they will be small and slow.
![]()
Leftover, you have an equation that states that the heat you transfer to the cube (per unit mass) causes the temperature change:
![]()
If you divide this by a time interval ∆t (note: lower-case t is used for time, T is temperature), gives an equation for temperature change with time:
![]()
The temperature of the air cube could be increased if there were a heat transfer into it. Heat flux is the rate of heat transfer through a surface over time, as illustrated in the below figure. The units of heat flux can be given as J·m-2·s-1, or W·m-2, because W = J·s-1. It is heat moving through an area over time.

Temperature could be increased by heat flux into the cube of air, and could also be decreased by heat flux out of the cube. For example, if there is a net heat flux into a cube of air there will be a net heating effect because heat will be transferred into the cube more quickly than it will leave the cube.
Earth’s Surface Budget
Fluxes are defined as positive for heat moving upward. In addition to the net radiation (F*) from shortwave and longwave radiation, the fluxes include:
F* = the net radiation between the surface and atmosphere, defined above;
FH = effective surface turbulent heat flux (sensible heat flux, SH);
FE = effective surface latent heat flux, caused by evaporation or condensation (latent heat flux, LH); and
FG = molecular heat conduction to/from deeper below the surface, basically heat being conducted from nearby molecules.
All of these fluxes have to balance.
![]()
Over the course of a day, the relative contributions from the different terms in the surface heat budget vary. The below figure shows the four terms as they vary over a moist surface during one average day. Notice the yellow line first. There is negative F* through most of the day as the surface gains heat from the excess shortwave radiation. At night, F* is positive as the surface radiates infrared radiation upward away from the surface. This is similar to FG, where heat is transferred downward into the ground during the day and upward at night. However FH and FE have opposite signs from F* and FG. The surface sensible heat flux, FE and surface latent heat flux, FH are positive during the day as convection and evaporation draws heat upward away from the surface.

Now let’s look at how the fluxes vary instantaneously over a moist (a, b) or dry (c, d) surface during the day (a, c) and night (b, d). The size of each arrow corresponds to the strength of each type of flux.

In the above image, note the difference between (a) and (c). Both have a large incoming radiation F*, but the moist surface (a) has a larger latent heat flux FE as compared to the dry desert in (c) with a larger sensible heat flux FH. This shows that heat is transferred from the ground to the atmosphere through evaporation when the surface is moist, but through convection when the surface is dry.
The Bowen ratio helps to distinguish various types of surfaces. The Bowen ratio is defined as
![]()
the ratio between the sensible heat flux and the latent heat flux. Moist surfaces have a small Bowen ratio because latent heating dominates over sensible heating while dry surfaces have a large Bowen ratio because sensible heating dominates over latent heating.
Remember our learning goals for this chapter:
- Define and describe four methods of energy transfer
- Describe the change in energy associated with changes in water state
- Define and apply the first law of thermodynamics
- Differentiate Eulerian and Lagrangian frameworks
- Describe the importance of the dry adiabatic lapse rate, and recall what sets its constant value in the atmosphere
- Compute potential temperature and apply the conserved variable approach
- Draw a diagram of surface heat fluxes and Earth’s radiation budget
- Compute the Bowen ratio, and define latent and sensible heat flux
Do you feel comfortable with all of the goals?
Additional Information
Temperature has been historically measured by a thermometer like the one below. A bulb of expandable fluid grows or shrinks depending on the temperature, indicating the temperature by its top. Thermometers of this type are robust and cheap, but not easily automated.

Another type of early thermometer records the minimum and maximum with mercury (below). A marker is pushed upward (for the maximum) or downward (for the minimum).

Today, most thermometers are electrical. They use components that are temperature sensitive, like a temperature sensitive resistor or capacitor where the electrical current changes depending on temperature. Based on the electrical reading from the instrument, temperature can be automatically recorded.

This type of thermometer is used in radiosondes, which are launched on weather balloons for measuring temperature throughout the entire atmospheric column. We can observe in-situ as high as 50 km, halfway through the stratosphere with a radiosonde balloon.

Thermometers need to be correctly sheltered so that solar radiation does not affect the temperature reading. A shelter is typically placed at least 1 meter above the ground and covered in a white housing that blocks the sun but allows air to flow through like the one in the image.

A temperature reading simply gives the temperature of the air, but wind and humidity can affect the way the atmosphere feels to the human body. The below image shows a wind chill graph where the wind speed and the temperature gives an additional measure of temperature called the wind chill. When the air temperature is cold and the wind is high, the temperature feels even colder and can give a person standing outdoors frostbite within minutes (see the purple area below).

On the other hand when the air is humid, it feels hotter. This is because when the air contains more water vapor and surfaces (like your skin) cannot evaporate it efficiently. Unable to get rid of heat through the latent heat flux (LHF), the surface heats up. This is called the heat index. Notice that a temperature of 84°F at 90% relative humidity has a heat index of 98°F.

You have experienced at least one of these issues (wind chill or heat index) in your lifetime.
Chapter 3: Questions to Consider
- Read the following scenarios and chose the type of energy transfer described.
- Fill in the blanks to describe what happens when an air parcel rises.
- Earlier in the chapter, a brick being held up was described as having potential energy. If the brick weighs 3.5 kg and is being held 1.2 m off the ground, how much potential energy does it have? Remember that acceleration from gravity is 9.8 m⋅s-2.
- Mauna Kea, an inactive volcano on the island of Hawai’i, has an elevation of 13,803 ft at the summit. If a weather balloon measures the environmental lapse rate to be 6.5 K⋅km-1, how much cooler is the summit than the rest of the island at sea level?
- In the winter, it often snows on top of Mauna Kea. Explain how that’s possible despite the mountain’s tropical location using your result from the previous question.
Selected Practice Question Answers: