5. Power System
5.6 Consumable Power Storage

A Ragone Plot of electrochemical devices shows storage capacity against instantaneous power output. On the extremes, capacitors store little energy but discharge very quickly (useful for short but powerful electrical movement, like deployment mechanisms). Fuel cells store an immense amount of energy but output power at a low rate. The lines represent the relative time to get charged in or out of the device. Lithium batteries are a compromise between fuel cells and capacitors, charging and discharging at a relative time of less than an hour, making them ideal candidates for Low Earth Orbit spacecraft due to the temporal match in the orbital period. This section will give an overview of the various power storage options, their physical inner workings, and design considerations.
Fuel Cells
Solar-powered hydrogen fuel cell demo. By Philip Russell
Fuel cells convert the chemical energy of a fuel and an oxidizing agent into electricity. Commonly, the fuel is hydrogen and the oxidizing agent is oxygen. The chemical reaction is shown by:
![]()
Where ![]() are hydrogen molecules,
are hydrogen molecules, ![]() are oxygen molecules,
are oxygen molecules, ![]() O are water molecules, and e- is a free electron that can produce electricity or heat. The production of electricity is solely dependent on the availability of hydrogen and oxygen, so when the fuel is gone, the ability to produce electricity disappears as well. You’ll see that the reaction produces water that may be used as potable water for humans or recaptured in a closed system in storage. Water can be electrolyzed into
O are water molecules, and e- is a free electron that can produce electricity or heat. The production of electricity is solely dependent on the availability of hydrogen and oxygen, so when the fuel is gone, the ability to produce electricity disappears as well. You’ll see that the reaction produces water that may be used as potable water for humans or recaptured in a closed system in storage. Water can be electrolyzed into ![]() and
and ![]() molecules with excess power as a form of regenerated fuel. This regenerative process requires a separate power source, like solar cells, but in this way, fuel cells act as a power storage device, like a high-capacity battery. Hydrogen fuel cells are on the horizon for small satellite power generation technologies [SmallSat Institute].
molecules with excess power as a form of regenerated fuel. This regenerative process requires a separate power source, like solar cells, but in this way, fuel cells act as a power storage device, like a high-capacity battery. Hydrogen fuel cells are on the horizon for small satellite power generation technologies [SmallSat Institute].

As fuel cells are not common for small satellites yet, we will not delve deeply into the equations for developing and sizing your own fuel cell. If interested, refer to these useful fuel cell equations that relate to oxygen usage rate, air inlet flow rate, air exit flow rate, hydrogen usage, and the energy content of hydrogen, rate of water production, heat production [Wiley]. The stoichiometric reaction formulas yield the electrical power of the whole fuel cell stack and the average voltage of each cell in the stack. Voltage is typically 0.6 – 0.7 V per cell; 0.65V is a safe assumption when voltage is not explicitly given. The electrical power is either a given or estimated specification that is the most basic and important information about a fuel cell system. For reference, an experimental fuel cell from the University of Illinois that is based on hydrogen peroxide rather than water has demonstrated an energy density of over 1000 Whkg-1 with a theoretical limit of over 2580 Whkg-1, less than half the theoretical limit [SmallSat Institute]! The maximum theoretical efficiency of a complete fuel cell system based on a lower heating value of hydrogen is 228.6 kJ/mole ∕ 241.8 kJ/mole or 94.5% [NREL]. You can see that the technology development for fuel cells for space has a long way to go and might be an exciting technology mission for CubeSats. I would highly suggest diving deep into the other references and taking courses in chemical engineering if this technology piques your interest.

Capacitors

On the other side of the extreme on the Ragone plot is the capacitor, which supplies high power at high-energy efficiency but has low energy density. For comparison, lithium-ion cells have an energy density of 150 Wh/kg, and super-capacitors have between 5 – 30 Wh/kg [Underwood]. The difference between capacitors and batteries is that capacitors store energy in an electric field on conductive plates and batteries store energy in chemical form.
Capacitors can act as a consumable power source when charged and connected to a load, but also as an energy storage option when connected to a charging circuit. “Capacitors are commonly used in electronic devices to maintain power supply while batteries are being changed” [Wikipedia]. Taking advantage of the capacitor’s high current discharge, capacitors may also be used to power ordnance devices, separation mechanisms, or deployers. For power conditioning to be discussed in the power management section, capacitors can “smooth current fluctuations for signal or control circuits” [Wikipedia]. With the rise of small satellites, these capacitors may satisfy the minimal energy and power requirements of a spacecraft the size of a cracker [ChipSat].

Primary Batteries
Suggested Reading
Batteries operate by converting chemical potential energy to electrical energy. “Batteries powering satellites or spacecraft must be rugged enough to withstand the severe vibrations of launch. Once the craft is deployed, these batteries must operate in extreme conditions of heat and cold and solar radiation. And, they need to work in a vacuum without leaking or exploding. There are many types of batteries: carbon-zinc, lead-acid, nickel-cadmium, nickel-hydrogen, silver zinc, alkaline, and lithium-ion to name a few” [NASA]. There are two types of batteries: primary and secondary; secondary batteries will be discussed in the power storage section. Primary batteries are single-use and disposable, storing a finite amount of energy and discharging until energy can not be withdrawn. Although consumable, primary batteries are typically higher in energy density, which may be preferred for short space mission lifetimes for their reliability and simplicity. These batteries were used before solar panels were widely adopted and are still commonly used to power small probes sent to the surface of planetary bodies [Wikipedia]. The most recent mission to use primary batteries is MASCOT, a scientific asteroid-hopping rover deployed by Hayabusa2. You can see that MASCOT is a tiny scientific, self-contained package with no deployable mechanisms; a short and sweet mission powered by primary batteries.

Primary batteries are evaluated by their energy density, discharge rate, and temperature limits. For all primary and secondary batteries, “important cell properties, such as voltage, energy density, flammability, available cell constructions, operating temperature range and shelf life”, which is dictated by battery chemical compositions. When sizing the primary battery, the EPS system lead must consider that the primary battery contains the entire energy budget of the mission, unless there is another power source. Based on the power budget, the primary battery must be able to output power associated with a minimum discharge rate. The battery must also be operational or thermally regulated to survive the thermal environment throughout the mission.
Battery Terminology
- Ampere-hour (Ah) – TOTAL CAPACITY OF BATTERY (e.g. 40 Amps for 1 hr = 40 Ah)
- Depth of discharge (DOD) – percent of battery capacity used in discharge (75% DOD means 25% capacity remaining, DOD usually limited for long cycle life)
- % DOD =
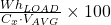
- WhLoad= Watt hours delivered to load = (Load in Watts) x (Duration in hours)
- C = Capacity of battery in amp-hours
-
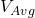 = Average battery discharge voltage
= Average battery discharge voltage
- For long cycle life, DOD may be limited to 50% to 75%
- Watt-hours – stored energy of the battery, equal to Ah capacity times average discharge voltage
- Charge rate – rate Ah which battery can accept a charge. Rule of thumb = Capacity (Ah)/15 hr
- Average discharge voltage – number of cells times cell discharge voltage (1.25 V for most cells)
