Private: Main Body
13 Race and Human Variation
Michael B. C. Rivera, Ph.D., University of Cambridge
Learning Objectives
- Review the illustrious and (at times) troubling history of “race” concepts.
- Recognize human diversity and evolution as the thematic roots of our discipline.
- Critique earlier “race” concepts based on overall human diversity being lower compared to other species and human genetic variation being greater within a population than between populations.
- Explain how biological variation in humans is distributed clinally and in accordance with both isolation-by-distance and Out-of-Africa models.
- Identify phenotypic traits that reflect selective and neutral evolution.
- Relate a more nuanced view of human variation with today’s ongoing bioanthropological research, implications for biomedical studies, applications in forensic anthropology, and sociopolitical/economic concerns.
Humans exhibit biological diversity. Cognitively, humans also have a natural desire to categorize objects and other humans in order to make sense of the world around them. Since the birth of the discipline of biological anthropology, we have been interested in studying how humans vary biologically and what the sources of this variation are. Before we tackle these big problems, this first begs the question: Why should we study human diversity?
There are certainly academic reasons for studying human diversity. First, it is highly interesting and important to consider the evolution of our species and how our biological variation may be similar to (or different from) that of other species of animals (e.g., other primates and apes). Such investigation can give us clues as to how unique we are as a biological organism in relation to the rest of the animal kingdom. Second, anthropologists study modern human diversity to understand how different biological traits developed over evolutionary time. If we are able to grasp the evolutionary processes that produce and affect diversity, we can make more accurate inferences about evolution and adaptation among our hominin ancestors, complementing our study of fossil evidence and the archaeological record. Third, as will be discussed in more detail later on, it is important to consider that biological variation among humans has biomedical, forensic, and sociopolitical implications. For these reasons, the study of human variation and evolution has formed the basis of anthropological inquiry for centuries and continues to be a major source of intrigue and inspiration for scientific research conducted today.
An even more important role of the biological anthropologist is to improve public understanding of human evolution and diversity, outside of academic circles. Terms such as race and ethnicity are used in everyday conversations and in formal settings within and outside academia. The division of humankind into smaller, discrete categories is a regular occurrence in day-to-day life. This can be seen regularly when governments acquire census data with a heading like “geographic origin” or “ethnicity.” Furthermore, such checkboxes and drop-down lists are commonly seen as part of the identifying information required for surveys and job applications.
According to the Oxford English Dictionary (2018), race is a term that should be used to describe one or more of the following:
- a major division of the human species based on particular physical characteristics;
- the biological origin of a group of people, or ancestry;
- the fact or condition of belonging to a racial division or group, or the social qualities associated with this;
- a group of people sharing the same culture and language;
- any group of people or things with a common feature or features;
- a population within a species that is distinct in some way, especially a subspecies.
So many various definitions for one word already suggests that perhaps the concepts or meanings behind biological diversity are complicated. Even though the terms race and ethnicity are used often in commonplace settings, there is no consensus among biological anthropologists as to what races are, whether they even exist, and, if they do, how the term should be applied to the human species meaningfully. If biological anthropologists cannot reach a consensus on how to view human diversity, how can we possibly expect there to be a clear perspective on the nature and causes of biological variation outside of scientific academia? Ideas about ethnicity that people hold have huge social and political impacts, and notions of race have been part of the motivation behind various forms of racism and prejudice today, as well as many wars and genocides throughout history. This is how the role of the biological anthropologist becomes crucial in the public sphere, as we may be able to debunk myths surrounding human diversity and shed light on how human variation is actually distributed worldwide for the non-anthropologists around us (Figure 13.1). Recent work in anthropological genetics has revealed the similarities amongst humans on a molecular level and the relatively few differences that exist between populations that one might be tempted to see as significantly distinctive.

Science communication and education that centers upon race and our species’ variation is interesting and important. Throughout this chapter, I will highlight how humans cannot actually be divided into discrete “races,” because most traits instead vary on a continuous basis and human biology is, in fact, very homogenous compared to the greater genetic variation we observe in other closely related species. The reason we know this now is thanks to technological developments that have taken place over the last 50 or so years. Molecular anthropology, or anthropological genetics, revolutionized and continues to add new layers to our understanding of human biological diversity and the evolutionary processes that gave rise to the patterns of variation we observe in contemporary populations. The study of human variation has not always been unbiased, and thinkers and scientists have always worked in their particular sociohistorical context. For this reason, this chapter opens with a brief overview of race concepts throughout history, many of which relied on unethical and unscientific notions about different human groups.
THE HISTORY OF “RACE” CONCEPTS
“Race” in the Classical Era
The earliest classification systems used to understand human diversity are evidenced by ancient manuscripts, scrolls, and stone tablets recovered through archaeological, historical, and literary research. The Ancient Egyptians had the Book of Gates, dated to the New Kingdom between 1550 B.C.E. and 1077 B.C.E (Figure 13.2). In one part of this tome dedicated to depictions of the underworld, scribes used pictures and hieroglyphics to illustrate a division of Egyptian people into the four categories known to them at the time: the Aamu (Asiatics), the Nehesu (Nubians), the Reth (Egyptians), and the Themehu (Libyans). Though not rooted in any scientific basis like our current understandings of human variation today, the Ancient Egyptians believed that each of these groups were made of a distinctive category of people, distinguishable by their skin color, place of origin, and even behavioral traits.


The Roman philosopher Pliny the Elder (23‒79 C.E.) also wrote about different groupings of people in his encyclopedia Naturalis Historia (Figure 13.3). In his opinion, all people fit under one of three categories: civilized peoples, barbarians, and monstrous individuals. Pliny the Elder’s work was deeply problematic. He believed that only Europeans were civilized and not monstrous-looking, while other groups of people lacked the ideal character and appearance. In both the cases of the Book of Gates and Naturalis Historia, the worldviews of those who wrote these volumes were also limited by how few and infrequent their encounters were with peoples elsewhere around the world—that is, those not residing in Europe, the Near East, or northern Africa. When faced with only the level of biological diversity they could see around them, distinguishing factors identified by these prominent thinkers relied simply on readily visible phenotypic traits, such as body size, skin color, and facial shape.
The most well-known of early documents is perhaps the Bible, where it is written that all humankind descends from one of three sons of Noah: Shem (the ancestor to all olive-skinned Asians), Japheth (the ancestor to pale-skinned Europeans), and Ham (the ancestor to darker-skinned Africans). Similar to the Ancient Egyptians, these distinctions were based on behavioral traits and skin color. More recent work in historiography and linguistics suggest that the branches of “Hamites,” “Japhethites,” and “Shemites” may also relate to the formation of three independent language groups some time between 1000 and 3000 B.C.E. With the continued proliferation of Christianity, this concept of approximately three racial groupings lasted until the Middle Ages and spread as far across Eurasia as crusaders and missionaries ventured at the time.

Finally, there is also the “Great Chain of Being,” conceived by ancient Greek philosophers like Plato (427‒348 B.C.E.) and Aristotle (384‒322 B.C.E.). They played a key role in laying the foundations of empirical science, whereby observations of everything from animals to humans were noted with the aim of creating taxonomic categories. Aristotle describes the Great Chain of Being as a ladder along which all objects, plants, animals, humans, and celestial bodies can be mapped in an overall hierarchy (in the order of existential importance, with humans placed near the top, just beneath divine beings) (Figure 13.4). Where he writes about humans, Aristotle expressed the belief that certain people are inherently (or genetically) more instinctive rulers, while others are more natural fits for the life of a worker or slave. Nowadays, based on research by biological anthropologists, we currently recognize that these early systems of classification and hierarchization are unhelpful in studying human biological diversity. Both behavioral traits and physical traits are coded for by multiple genes each, and how we exhibit those traits based on our genetics can vary significantly even between individuals of the same population.
“Race” during the Scientific Revolution
The 1500s and 1600s saw the beginnings of the “Scientific Revolution” in European societies, with thinkers like Copernicus, Galileo, and Da Vinci publishing some of their most important findings. While by no means the first or only scholars globally to use observation and experimentation to understand the world around them, early scientists living at the end of the medieval period in Europe increasingly employed more experimentation, quantification, and rational thought in their work. This is the main difference between the work of the ancient Egyptians, Romans, and Greeks, and that of workers like Isaac Newton and Carl Linnaeus in the 1600s and 1700s.

Linnaeus is the author of Systema Naturae (1758), in which he classified all plants and animals he could observe under the first formalized naming system known as binomial nomenclature (i.e., how all organisms can be named by their genus and species, such as Homo sapiens or Pan troglodytes) (Figure 13.5). What was most anthropologically notable about Linnaeus’s taxonomy was that he was one of the first to group humans with apes and monkeys, after noting the anatomical similarities between humans and nonhuman primates. Linnaeus viewed the world in line with essentialism, a concept which dictates that there are a unique set of characteristics that organisms of a specific kind must have—organisms would fall outside taxonomic categorizations if they lacked any of the required criteria.
Despite these useful contributions to the biological sciences, Linnaeus still subdivided the human species into four varieties, with overtly racist categories based on skin color and “inherent” behaviors. According to him, Africans are all “black-skinned” and ruled by an erratic nature; Native Americans are “red” in skin tone and ruled by habit; Asians are “yellow-” or “brown-skinned” and ruled by belief; and Europeans are “white” and regulated by custom. These standards for categorization imply that Europeans are governed by carefully considered culture and custom, unlike the unthinking Asians and Indigenous Americans in his framework who normally act out of “habit” or “belief.” Moreover, Linnaeus’s traditional ranking also places sub-Saharan, dark-skinned Africans inferior to the other three. Wrongly so, European scientists during this period were not aware of their own biases skewing their interpretations of biological diversity. The conclusions and claims they came to, consciously or subconsciously, often fit such an age when the superiority of European cultures over others was a pervasive idea throughout these scientists’ social and political lives.

Occurring alongside this Scientific Revolution was also the “Age of Discovery.” Although much of Eurasia was linked by spice and silk trading routes, the European colonial period between the 1400s and 1700s was marked by many new and intentionally violent encounters overseas (Figure 13.6). When Europeans arrived by ship on the shores of continents that were already inhabited, it was their first meeting with the indigenous peoples of the Americas and Australasia, who looked, spoke, and behaved differently from peoples with whom they were familiar. Building on the idea of species and “subspecies,” natural historians of this time invented the term race, from the French rasse meaning “local strain.” The idea behind this terminology was rooted in the observation that geography plays a significant role in producing the biological traits we observe today. Naturalists like Comte de Buffon and Johann Blumenbach did believe that all people have a single origin, but they also believed that differences in environment could lead to biological changes between different groups of people (i.e., races). However, as they had no understanding of genetics, they were incorrect in assuming that factors such as skin color could change in a single lifetime depending on climate and diet and, essentially, behavior. Again, while drawing links between external physical characteristics and behavior is not scientific, differences in both were used to justify the Othering of “nonwhite” cultures. Establishing “otherness” and “inferiority” in other people’s cultures was necessary at the time for colonialists to enforce European domination and the subordination of non-European people. Without genetic technologies, little was known at the time about the hereditary or evolutionary basis of skin color having little to do with innate differences between various “races.”
Another such scientist at the time, Johann Friedrich Blumenbach (1752‒1840), classified humans into five races based on his observations of cranial form variation as well as skin color. He thus dubbed the “original” form of the human cranium the “Caucasian” form, with the idea that the ideal climate conditions for early humans would have been in the Caucasus region near the Caspian Sea. The key insight Blumenbach presented was that human variation in any particular trait should be more accurately viewed as falling along a gradation (Figure 13.7). While some of his theories were correct according to what we observe today with more knowledge in genetics, workers like him and Buffon believed erroneously that human “subspecies” were “degenerated” or “transformed” varieties of an ancestral Caucasian or European race. According to them, the Caucasian cranial dimensions were the least changed over human evolutionary time, while the other skull forms represented geographic variants of this “original.” As will be discussed in greater detail later in this chapter, we have genetic and craniometric evidence for sub-Saharan Africa being the origin of the human species instead. Based on work that shows how most biological characteristics are coded for by nonassociated genes, it is not reasonable to draw links between individuals’ personalities and their skull shapes.

“Race” and the Dawn of Scientific Racism
Between the 1800s and mid-1900s, and contrary to what you might expect, an increased use of scientific methods to justify racial schemes developed in scholarship. Differing from Blumenbach and Buffon’s views in earlier centuries, which saw all humans as environmentally deviated from one “original” humankind, classification systems after 1800 became more polygenetic (viewing all people as having separate origins) rather than monogenetic (viewing all people as having a single origin). Instead of moving closer to our modern-day understandings of human diversity, there was increased support for the notion that each race was created separately and with different attributes (intelligence, temperament, and appearance).
The 1800s were an important precursor to modern biological anthropology as we know it, given that the scientific measurement of human physical features (anthropometry) truly became popularized then. However, whether it was skin color, skull shape, or observations of behavior being analyzed as the data, empirical studies in the 1800s pushed the idea even further that Europeans were culturally and biologically superior. The leading figures in craniometry at this time, focusing on measurements of the skull, were also linked heavily with powerful individuals and wealthy sociopolitical institutions and financial bodies. Therefore, polygenetic ways of thinking were particularly influenced by sociohistorical and economic factors at the time. Theories in support of hierarchical racial schemes certainly helped continue the exploitative and unethical transatlantic slave trade between the 1500s and 1800s by justifying the transport and enslavement of African people on a “scientific” basis.
While considered one of the pioneers of American “physical” anthropology, Samuel George Morton (1799‒1851) was a scholar who had a large role in 1800s scientific racism. By measuring cranial size and shape, he calculated that “Caucasians,” on average, have greater cranial volumes than other groups, such as the Native Americans and “Negros.” Today, we know that cranial size variation depends on such factors as Allen’s and Bergmann’s rules, which give the more likely explanation for the largest heads being found in people living among colder regions (i.e., Europeans) being climatic adaptation (Beals et al. 1984). In colder environments, it is advantageous for those living there to have larger and rounder heads because they conserve heat more effectively than slenderer heads (Beals et al. 1984).
Morton went on to write in his publication Crania Americana (1839) a number of views that fit with a concept called biological determinism. The idea behind biological determinism is that an association exists between people’s physical characteristics and their behavior, intelligence, ability, values, and morals. If the idea is that some groups of people are essentially superior to others in cognitive ability and temperament, then it is easier to justify the unequal treatment of certain groups based on outward appearances. Based on his cranial measurements and observations of human nature, Morton claimed that Europeans were the most intelligent and “well-proportioned,” while Asians were not fit for leadership and had short attention spans, Native Americans were slow in acquiring knowledge and fond of war, and Africans were superstitious, uninventive, and “barbarous.”
Another such problematic thinker was Paul Broca (1824‒1880), after which a region of the frontal lobe related to language use is named (Broca’s area). Influenced by Morton, he likewise claimed that internal skull capacities could be linked with skin color and cognitive ability. Considering his data taken from different parts of the globe, Broca thought that factors such as gender, education, and social status could have an influence on brain size for different groups, purporting that men had larger brains than women and that “eminent” men were superior to men of “mediocre talent.” He went on to justify the European colonization of other global territories by purporting it was noble for a biologically more “civilized” population to improve the “humanity” of more “barbaric” populations. Today, these theories of Morton, Broca, and others like them are known to have no scientific basis. If we could speak with them today, they would likely try to emphasize that their conclusions were based on empirical evidence and not a priori reasoning. However, we now can clearly see that their reasoning was biased and affected by prevailing societal views at the time.
“Race” and the Beginnings of Physical Anthropology
In the early 20th century, we saw a number of new figures coming into the science of human variation and shifting the theoretical focuses within. Most notably, these included Aleš Hrdlička and Franz Boas.

Aleš Hrdlička (1869‒1943) was a Czech anthropologist who moved to the United States. In 1903, he established the physical anthropology section of the National Museum of Natural History (Figure 13.8). In 1918, he founded the American Journal of Physical Anthropology, one of the foremost scientific journals disseminating bioanthropological research still today. As part of his work and the scope of the journal, he differentiated “physical anthropology” from other kinds of anthropology—he wrote that physical anthropology is “the study of racial anatomy, physiology, and pathology” and “the study of man’s variation” (Hrdlička 1918). In some ways, although the scope and technological capabilities of biological anthropologists have changed significantly, Hrdlička established an area of inquiry that has continued and prospered for over a hundred years.
Franz Boas (1858‒1942) was a German American anthropologist who established the four-field anthropology system in the United States and founded the American Anthropological Association in 1902. He argued that the scientific method should be used in the study of human cultures and the comparative method for looking at human biology worldwide. Boas’s specialization was in the study of skull dimensions with respect to race. After a long-term research project, he demonstrated how cranial form was highly dependent on cultural and environmental factors and that human behaviors were influenced primarily not by genes but by social learning. He wrote in one essay for the journal Science: “While individuals differ, biological differences between races are small. There is no reason to believe that one race is by nature so much more intelligent, endowed with great willpower, or emotionally more stable than another, that the difference would materially influence its culture” (Boas 1931:6). This conclusion directly contrasted with the theories of the past that relied on biological determinism. Biological anthropologists today have found evidence that corroborates Boas’s explanations: societies do not exist on a hierarchy or gradation of “civilizedness” but instead are shaped by the world around them, their demographic histories, and the interactions they have with other groups.
The first half of the 1900s still involved some research that was essentialist and focused on proving racial determinism. Anthropologists like Francis Galton (1822‒1911) and Earnest A. Hooton (1887‒1954) created the field of eugenics as an attempt to formalize the social scientific study of “fitness” and “superiority” among members of 19th-century Europe. As a way of “dealing with” criminals, diseased individuals, and “uncivilized” people, eugenicists recommended prohibiting parts of the population from being married and sterilizing these members of society so they could no longer procreate (Figure 13.9). They instead encouraged “reproduction in individual families with sound physiques, good mental endowments, and demonstrable social and economic capability” (Hooton 1936). In the 1930s, Nazi Germany used this false idea of there being “pure races” to highly destructive effect. The need to be protected against admixture from “unfit” groups was their justification for their blatant racism and purging of citizens that fell under their subjective criteria.
Shortly after World War II and the Nazi Holocaust, the full extent of essentialist, eugenicist thinking became clear. Social constructions of race, and the notion that you could predict psychological or behavioral traits based on external appearance, had become unpopular both within and outside the discipline. It was up to those in the field of physical anthropology at the time to separate physical anthropology from race concepts that supported unscientific and socially damaging agendas. This does not mean that there are no physiological or behavioral differences between different members of the human species. However, going forward, a number of physical anthropologists saw human biological variation as more complicated than simple typologies could describe.
HUMAN VARIATION IN BIOLOGICAL ANTHROPOLOGY TODAY
“Populations” Instead of “Races”

After 1950, replacing the concept of “race” as a unit of diversity was the “population.” This was outlined by those pioneering the “new physical anthropology,” such as Sherwood Washburn, Theodosius Dobzhansky, and Julian Huxley, who borrowed this way of framing human groups from contemporary population geneticists (Figure 13.10). “Races” were then defined simply as populations that differ in the frequency of some gene or genes. And, on the other hand, a “population” is a group of individuals potentially capable of or actually interbreeding due to shared geographic proximity, language, ethnicity, culture, and/or values. Put another way, a population is a local interbreeding group with reduced gene flow between themselves and other groups of humans. Members of the same population may be expected to share many genetic traits (and, as a result, many phenotypic traits that may or may not be visible outwardly).

Thinking of humans in terms of populations was part of Julian Huxley’s (1942) “Modern Synthesis”—so named because it helped to reconcile two fundamental principles about evolution that had not been made sense of together before (Figure 13.11). As discussed in Chapter 3, Gregor Mendel (1822‒1884) was able to show that inheritance was mediated by discrete particles (or genes) and not blended in the offspring. However, it was difficult for some 19th-century scientists to accept this model of genetic inheritance at the time because much of biological variation appeared to be continuous and not particulate (take skin color or height as examples). In the 1930s, it was demonstrated that traits could be polygenic and that multiple alleles could be responsible for any one phenotypic trait, thus producing the continuous variation in traits such as eye color that we see today. Thus, Huxley’s “Modern Synthesis” outlines not only how human populations are capable of exchanging genes at the microevolutionary level but also how multiple alleles for one trait (polygenic exchanges) can cause gradual macroevolutionary changes.
Human Variation Is Clinal/Continuous (Not Discrete)
Human diversity cannot be broken into discrete “races,” because most physical traits vary on a continuous or “clinal” basis. One obvious example of this is how human height does not only come in three values (“short,” “medium,” and “tall”) but instead varies across a spectrum of vertical heights achievable by humans all over the world. (However, this is with the only difference being the huge divergence in how factors like body size and traits such as skin color have been viewed and used sociopolitically as a way of separating people throughout history.) The need to shift from typological “race” categories to a more nuanced understanding of continuously variable populations was realized by anthropologists working in the 1960s and 1970s who shifted their focus toward the study of individual traits rather than the study of groups (populations, races). Systematic evaluations of global biological variation in humans only began then, when large numbers of genetic loci for large numbers of samples were sampled from human populations distributed worldwide. It was during the 1960s that “clines” in human genetic variation were first identified.
Frank B. Livingstone (1928‒2005) wrote: “There are no races, only clines” (1962). A cline is a gradation in the frequency of an allele/trait between populations living in different geographic regions. In order to study human traits that are clinally distributed, it is often required to perform genetic testing to uncover the true frequencies of an allele or trait across a certain geographic space. One easily visible example of a clinal distribution seen worldwide is the patterning of human variation in skin color. Whether in southern Asia, sub-Saharan Africa, or Australia, dark brown skin is found. Paler skin tones are found in higher-latitude populations such as those who have lived in areas like Europe, Siberia, and Alaska for millennia. Skin color is easily observable as a phenotypic trait exhibiting continuous variation.
A clinal distribution still derives from genetic inheritance, but clines often correspond to some gradually changing environmental factor. Clinal patterns arise when selective pressures in one geographic area differ from those in another as well as when people procreate and pass on genes together with their most immediate neighbors. There are several mechanisms, selective and neutral, that can lead to the clinal distribution of an allele or a biological trait. Natural selection is the mechanism that produced a global cline of skin color, whereby darker skin color protects equatorial populations from high amounts of UV radiation; there is a transition of lessening pigmentation in individuals that reside further and further away from the tropics (Jablonski 2004; Jablonski and Chaplin 2000) (Figure 13.12). The ability and inability to digest lactose (milk sugar) among different world communities varies according to differential practices and histories of milk and dairy product consumption (Gerbault et al. 2011; Ingram et al. 2009). Where malaria seems to be most prevalent as a disease stressor on human populations, a clinal gradient of increasing sickle cell anemia experience toward these regions has been studied extensively by genetic anthropologists (Luzzatto 2012). Sometimes culturally defined mate selection based on some observable trait can lead to clinal variation between populations as well.

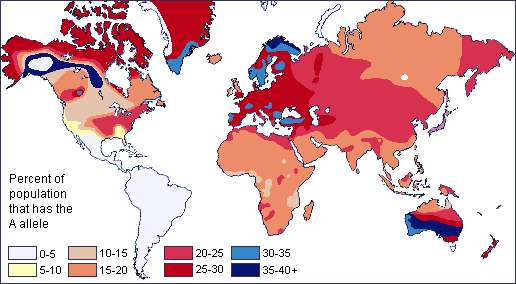
Two neutral microevolutionary processes that may produce a cline in a human allele or trait are gene flow and genetic drift. The ways in which neutral processes can produce clinal distributions is seen clearly when looking at clinal maps for different blood groups in the human ABO blood group system (Figure 13.13). For instance, scientists have identified an east-to-west cline in the distribution of the blood type B allele across Eurasia. The frequency of B allele carriers decreases gradually westward when we compare the blood groups of East and Southeast Asian populations with those in Europe. This shows how populations residing nearer to one another are more likely to interbreed and share genetic material (i.e., undergo gene flow). We also see 90%‒100% of native South American individuals, as well as between 70%‒90% of Aboriginal Australian groups, carrying the O allele (Mourant et al. 1976). These high frequencies are likely due to random genetic drift and founder effects, in which population sizes were severely reduced by the earliest O allele-carrying individuals migrating into those areas. Over time, the O blood type has remained predominant.


The Apportionment of Human Variation: Genetic Diversity Is Greater Within-Group Than Between-Groups
One problem with race-based classifications is they relied on an erroneous idea that people within a typological category were more similar to each other than they were to people in other groups. In other words, “race” concepts were predicated on the notion that individuals with particular characteristics would share more similar genes with each other within a particular “race” and share less with individuals of other “races” possessing different traits and genetic makeups. However, since around 50 years ago, scientific studies have shown that the majority of human genetic differences worldwide exist within groups (or “races”) individually rather than between groups.
Richard Lewontin (1929‒) is a biologist and evolutionary geneticist who authored a paper evaluating where the total genetic variation in humans lies. This article, titled “The Apportionment of Human Diversity” (Lewontin 1972), addressed the following question: On average, how genetically similar are two randomly chosen people from the same group when compared to two randomly chosen people from different groups? Lewontin studied this problem by using genetic data. He obtained data for a large number of different human populations worldwide using 17 genetic markers (including alleles that code for various important enzymes and proteins, such as blood-group proteins). The statistical analysis he ran used a measure of human genetic differences in and among populations known as the fixation index (FST). Technically, FST can be defined as the proportion of total genetic variance within a subpopulation relative to the total genetic variance from an entire population. Therefore, FST values range from 0 to 1 (or, sometimes you will see this stated as a percentage between 0% and 100%). The closer the FST value of a population (e.g., the world’s population) approaches 1, the higher the degree of genetic differentiation among subpopulations relative to the overall population. In his paper, Lewontin (1972) identified that most of human genetic differences (85.4%) were found within local subpopulations (e.g., the Germans or Easter Islanders), whereas 8.3% were found between populations within continental human groups, and 6.3% were attributable to traditional “race” groups (e.g., “Caucasian” or “Amerind”). These findings have been important for scientifically rejecting the existence of biological races (Long and Kittles 2008).
In 2002, another landmark article by Noah Rosenberg and colleagues (2002) explored worldwide human genetic variation using an even-greater genetic data set. They used 377 highly variable markers in the human genome and sampled from 1,056 individuals representative of 52 populations. The markers chosen for study were not ones that code for any expressed genes. Because these regions of the human genome were made of unexpressed genes, we may understand these markers as neutrally derived (as opposed to selectively derived) as they do not code for functional advantages or disadvantages. These neutral genetic markers likely reflect an intricate combination of regional founder effects and population histories. Analyses of these neutral markers allowed scientists to identify that a majority of global genetic variance (93%‒95%) can be accounted for by within-population differences at the 377 genetic loci, while only a small proportion of genetic variance (3%‒5%) can be attributed to differences among major groups (Rosenberg et al. 2002). Like Lewontin’s (1972) findings, this lends support to the theory that distinct biological races do not exist, even though misguided concepts of race may still have real social and political consequences.
Biological Data Fit Isolation-By-Distance and Out-of-Africa Models
One further note is that while the world’s population may be genetically divided into “groups,” “subsets,” “clumps,” or “clusters” that reflect some degree of genetic similarity, it is more likely that these identifiable clusters reflect genetic or geographic distances—either with gene flow facilitated by proximity between populations or impeded by obstacles like oceans or environmentally challenging habitats (Rosenberg et al. 2005). Sometimes, inferred clusters using multiple genetic loci are interpreted by non-geneticists literally as “ancestral populations.” However, it would be wrong to assume from these genetic results that highly differentiated and “pure” ancestral groups ever existed. These groupings reflect differences that have arisen over time due to clinal patterning, genetic drift, and/or restricted or unrestricted gene flow (Weiss and Long 2009). The clusters identified by scientists are arbitrary and the parameters used to split up the global population into groups is subjective and dependent on the particular questions or distinctions being brought into focus (Relethford 2009).

Additionally, research on worldwide genetic diversity has shown that human variation decreases with increasing distance from sub-Saharan Africa, where there is evidence for this vast region being the geographical origin of anatomically modern humans ( Liu et al. 2006; Prugnolle et al. 2005) (Figure 13.14). Genetic differentiation decreases in human groups the further you sample data from relative to sub-Saharan Africa because of serial founder effects (Relethford 2004). Over the course of human colonization of the rest of the world outside Africa, populations broke away in expanding waves across continents into western Asia, then Europe and eastern Asia, followed by Oceania and the Americas. As a result, founder events occurred whereby genetic variation was lost, as the colonization of each new geographical region involved a smaller number of individuals moving from the original larger population to establish a new one (Relethford 2004). The most genetic variation is found across populations residing in different parts of sub-Saharan Africa, while other current populations in places like northern Europe and the southern tip of South America exhibit some of the least genetic differentiation relative to all global populations.
Besides fitting nicely into the Out-of-Africa model, worldwide human genetic variation conforms to an isolation-by-distance model, which predicts that genetic similarity between groups will decrease exponentially as the geographic distance between them increases. This is because of the greater and greater restrictions to gene flow presented by geographic distance, as well as cultural and linguistic differences that occur as a result of certain degrees of isolation. Since genetic data conform to isolation-by-distance and Out-of-Africa models, these findings support the abolishment of “race” groupings. This research demonstrates that human variation is continuous and cannot be differentiated into geographically discrete categories. There are no “inherent” or “innate” differences between human groups; instead, variation derives from some degree of natural selection, as well as neutral processes like population bottlenecking (Figure 13.15), random mutations in the DNA, genetic drift, and gene flow through between-mate interbreeding.

Humans Have Higher Homogeneity Compared to Many Other Species
An important fact to bear in mind is that humans are 99.9% identical to one another. This means that the apportionments of human diversity discussed above only concern that tiny 0.1% of difference that exists between all humans globally. Compared to other mammalian species, including the other great apes, human diversity is remarkably lower. This may be surprising given that the worldwide human population has already exceeded seven billion, and, at least on the surface level, we appear to be quite phenotypically diverse. Molecular approaches to human and primate genetics tells us that external differences are merely superficial. For a proper appreciation of human diversity, we have to look at our closest relatives in the primate order and mammalian class. Compared to chimpanzees, gibbons, and even gray wolves and giant pandas, humans have remarkably low average genome-wide heterogeneity.

When we look at chimpanzee genetic diversity, it is fascinating that western, central, eastern, and Cameroonian chimpanzee groups have substantially more genetic diversity between them than large global samples of human DNA (Bowden et al. 2012) (Figure 13.16). This is surprising given that all of these chimpanzee groups live relatively near one another in Africa, while measurements of human genetic diversity have been conducted using samples from entirely different continents. First, geneticists suppose that this could reflect differential experiences of the founder effect between humans and chimpanzees. Because all non-African human populations descended from a small number of anatomically modern humans who left Africa, it would be expected that all groups descended from that smaller ancestral group would be similar genetically. Second, our species is really young, given that we have only existed on the planet for around 150,000 to 300,000 years. This gave humans little time for random genetic mutations to occur as genes get passed down through genetic interbreeding and meiosis. Chimpanzees, however, have inhabited different ecological niches, and less interbreeding has occurred between the four chimpanzee groups over the past six to eight million years compared to the amount of gene flow that occurred between worldwide human populations (Bowden et al. 2012).
Recent advances have now enabled the attainment of genetic samples from the larger family of great apes and the evaluation of genetic diversity among bonobos, orangutans, and gorillas alongside that of chimpanzees and humans (Prado-Martinez et al. 2013). Collecting such data and analyzing primate genetic diversity has been important not only to elucidate how different ecological, demographic, and climatic factors have shaped our evolution but also to inform upon conservation efforts and medical research. Genes that may code for genetic susceptibilities to tropical diseases that affect multiple primates can be studied through genome-wide methods. Species differences in the genomes associated with speech, behavior, or cognition could tell us more about how human individuals may be affected by genetically derived neurological or speech-related disorders and conditions (Prado-Martinez et al. 2013; Staes et al. 2017). In 2018, a great ape genomic study also reported genetic differences between chimpanzees and humans related to brain cell divisions (Kronenberg et al. 2018). From these results, it may be inferred that cognitive or behavioral variation between humans and the great apes might relate to an increased number of cortical neurons being formed during human brain development (Kronenberg et al. 2018). Comparative studies of human and nonhuman great ape genetic variation highlight the complex interactions of population histories, environmental changes, and natural selection between and within species. When viewed in the context of overall great ape diversity, we may reconsider how variable the human species is relatively and how unjustified previous “race” concepts really were.
Phenotypic Traits That Reflect Neutral Evolution
Most human traits are non-concordant. “Non-concordance” is a term used to describe how biological traits vary independent of each other—that is, they don’t get inherited in a correlative manner with other genetically controlled traits. For example, if you knew an individual had genes that coded for tall height, you would not be able to predict if they are lighter-skinned or have red hair. Depending on the trait being observed, different patterns of phenotypic variation may be found within and among groups worldwide. In this subsection, some phenotypic traits that reflect the aforementioned patterns of genetic variation will be discussed.

Looking beyond genetic variation briefly, recent studies have revisited biological anthropology’s earlier themes of externally observable traits, such as skull shape (Figure 13.17). In the last 20 or so years, anthropologists have evaluated the level to which human cranial shape diversity reflects the results from genetic markers, such as those used previously to fit against Out-of-Africa models (Relethford 2004) or those used in the apportionment of human diversity between and within groups (Lewontin 1972; Rosenberg et al. 2002). Using larger sample sizes of cranial data collected from thousands of skulls worldwide and a long list of cranial measurements, studies demonstrate a similar decrease in diversity with distance from Africa and show that a majority of cranial variation occurs within populations rather than between populations (Betti et al. 2009; Betti et al. 2010; Manica et al. 2007; Relethford 2001; von Cramon-Taubadel and Lycett 2008). The greatest cranial diversity is found among skulls of sub-Saharan African origin, while the least variation is found among populations inhabiting places like Tierra del Fuego at the southern tip of Argentina and Chile. While ancient and historical thinkers previously thought “race” categories could reasonably be determined based on skull dimensions, modern-day analyses using more informative sets of cranial traits simply show that migrations out of Africa and the relative distances between populations can explain a majority of worldwide cranial diversity (Betti et al. 2009).

This same patterning in phenotypic variation has even been found in studies examining shape variation of the pelvis (Betti et al. 2013; Betti et al. 2014), the teeth (Rathmann et al. 2017), and the human bony labyrinth of the ear (Ponce de León et al. 2018) (Figure 13.18). The skeletal morphology of these bones still varies worldwide, but a greater proportion of that variation can still be attributed to the ways in which human populations migrated across the world and exchanged genes with those closer to them rather than those further away. Human skeletal variation in these parts of the body is continuous and non-discrete. Given the important functions of the cranium and these other skeletal parts, we may infer that the genes that underpin their development have been relatively conserved by neutral evolutionary processes such as genetic drift and gene flow. It is also important to note that while some traits such as height, weight, cranial dimensions, and body composition are determined, in part, by genes, the underlying developmental processes behind these traits are underpinned by complex polygenic mechanisms that have led to the continuous spectrum of variation in such variables among modern-day human populations.
Phenotypic Traits That Reflect Natural Selection
Even though 99.9% of our DNA is the same between all humans worldwide, and many traits reflect neutral processes, there are parts of that remaining 0.1% of the human genome that code for individual and regional differences. Similarly to craniometric analyses that have been conducted in recent decades, human variation in skin color has also been reassessed using new methods and in light of greater knowledge of biological evolution.
New technologies allow scientists to use color photometry to sample and quantify the visible wavelength of skin color, in a way 19th- and 20th-century readers could not. In one report, it was found that 87.9% of global skin color variation can be attributed to genetic differences between groups, 3.2% to those among local populations within regions, and 8.9% within local populations (Relethford 2002). This apportionment differs significantly and is the reverse situation found in the distribution of genetic differences we see when we examine genetic markers such as blood type–related alleles. However, this pattern of human skin color worldwide is not surprising, given that we now understand that past selection has occurred for darker skin near the equator and lighter skin at higher latitudes (Jablonski 2004; Jablonski and Chaplin 2000). While most genetic diversity reflects neutral variation due to population migrations, geographic isolation, and restricted gene flow dynamics, some human genetic/phenotypic diversity is best explained as local adaptation to environmental conditions (i.e., selection). Given that skin color variation is atypical compared to other genetic markers and biological traits, this, in fact, goes against earlier “race” typologies. This is because recent studies ironically show how so much of genetic variation relates to neutral processes, while skin color does not. It follows that skin color cannot be viewed as useful in making inferences about other human traits.
On top of social implications, the quantification and interpretation of human variation has important medical and clinical applications (National Research Council Committee on Human Genome Diversity 1997). For instance, large-scale genomic studies sampling from human populations distributed worldwide have produced detailed knowledge on variation in disease resistance or susceptibility between and within populations. If we think about drug companies who develop medicines for African American patients particularly, the genetic diversity in predispositions to disease and/or good health is likely higher among people of African descent than these pharmaceutical businesses have taken into account. Through targeted sampling of various world groups, clinical geneticists may also identify genetic risk factors of certain common disorders such as chronic heart disease, asthma, diabetes, autoimmune diseases, and behavioral disorders. Having an understanding of population-specific biology is crucial in the development of therapies, medicines, and vaccinations, as not all treatments may be suitable for for every human, depending on their genotype. During diagnosis and treatment, it is important to have an evolutionary perspective on gene-environment relationships in patients. Typological concepts of “race” are not useful, given that most racial groups (whether self-identified or not) popularly recognized lack homogeneity and are, in fact, variable. Cystic fibrosis, for instance, occurs in all world populations but can often be underdiagnosed in populations of African ancestry because it is thought of as a “white” disease (Yudell et al. 2016).

Lastly, assignments of “race” to human remains is a common practice in forensic anthropology, especially in the United States and other worldwide contexts where bones are recovered and associated with criminal investigations (Figure 13.19). Forensic anthropologists have ascribed “race” or ancestry to sets of skeletons thanks to scientific research that has attempted to divide up different human groups into culturally constructed categories based on biologically “discrete” assortments (Sauer 1992). Rather than focusing on the neutral or selective causes of human biological variation, the concentration in forensic anthropology centers upon how probabilistic it may be to assign bones of certain dimensions to one of several identified racial categories. Forensic anthropologists do not agree with the typological “race” concepts of the past but, instead, root their racial categorization in methods of probability estimation (Sauer 1992). Based on many samples of skeletons from different world regions, statistical tests (such as discriminant function analysis) allow them to distinguish how likely certain skeletal dimensions may predict geographic ancestry.
It is important to remember that while it is possible to determine geographic origin (or ancestry) based on skull morphology, again, the amount of craniometric distinctiveness required to distinguish whether a cranium belongs to one group or another will make for arbitrary decisions (Relethford 2009). Individuals can vary in their skeletal dimensions by continental origin, country origin, regional origin, sex, age, environmental factors, and the time period in which they lived, making it difficult to assign individuals to particular categories in a completely meaningful way (Ousley et al. 2009). When forensic reports and scientific journal articles give an estimation of ancestry, it is crucial to keep in mind that responsible assignments of ancestry will be done through robust statistical testing and stated as a probability estimate. Today, we also live in a more globalized world where a skeletal individual may have been born originally to parents of two separate traditional racial categories. In contexts of great heterogeneity within populations, this definitely adds difficulty to the work of forensic scientists and anthropologists preparing results for the courtroom.
TALKING ABOUT HUMAN BIOLOGICAL VARIATION GOING FORWARD
To conclude, utilizing races to describe human biological variation is not accurate or productive. Using a select few hundred genetic loci, or perhaps a number of phenotypic traits, it may be possible to assign individuals to a geographic ancestry. However, what constitutes a bounded genetic or geographical grouping is both arbitrary and potentially harmful owing to ethical and historical reasons (see Chapter 3 for more on the issues with commercial ancestry tests, for example). The discipline of biological anthropology has moved past typological frameworks that shoehorn continuously variable human populations into discrete and socially constructed subsets. Improvements in the number of markers, the genetic technologies used to study variation, and the number of worldwide populations sampled have led to more nuanced understandings of human diversity. It is of utmost importance that scientists and non-scientists, in theory, have each of the following clarified:
- Today, we refer to different local human groups as “populations.” What constitutes a population is carefully defined in scientific reports based on some geographical, linguistic, or cultural criteria and some degree of relativity to other closely or distantly related human groups.
- Humans have significantly less genetic diversity than other primates and mammals, and all human beings on Earth share 99.9% of their overall DNA. Some of the remaining 0.1% of human variation varies on a clinal or continuous basis, such as can be seen when looking at ABO blood type polymorphisms worldwide.
- Many biological characteristics in humans are actually determined non-concordantly and/or polygenically. Therefore, superiority or inferiority in human behavior or body form cannot justifiably be linked to fixed and innate differences between groups.
- Genetic distances are correlated with geographic distances among the global human population. This is especially apparent when we consider that genetic diversity is highest in sub-Saharan Africa, and average genetic heterogeneity decreases in populations further away from the African continent in accordance with the migratory history of anatomically modern Homo sapiens.
- The effects of gene flow, genetic drift, and population bottlenecking are reflected in some phenotypic traits, such as cranial shape.
- Other traits, like skin color and lactase persistence, we recognize to be the product of many millennia of natural selective pressures influencing human biology from the external environment.
When taken altogether, genetic analyses of human diversity do not support 20th-century (or even earlier) concepts of race. In discussions about human diversity, each of these genomic results help clarify for all conversationalists how biological variation is distributed across the human population today. Taking care to think about and debate the nature of human variation is important, because although the effects and events that produced genetic differences among groups occurred in the ancient past, sociocultural concepts about race and ethnicity continue to have real social, economic, and political consequences in the modern era.
Beyond talking about diversity in the university setting, it is important that teachers, researchers, and students of anthropology recognize and assume the responsibility of influencing public perspectives of human diversity. Race-based classification systems were developed during the colonial era, transatlantic slave trade, and Scientific Revolution by some of the earliest scholars whom we may call the first “anthropologists” and students of humankind’s variation. Unfortunately, some of their ideas put forward have persisted and evolved into present-day lived realities. Some of today’s politicians and socioeconomic bodies have racially charged agendas that promote racism or certain kinds of economic or racial inequalities. As anthropologists, we must acknowledge that while human “races” are not a biological reality, their status as a (misguided) social construction does have real consequences for many people (Antrosio 2011). In other words, while “race” is a sociocultural invention in some people’s minds, the treatment different individuals receive due to their perceived “race” can have significant financial, emotional, sociopolitical, and physiological costs. But assuming a “color-blind” position when it comes to the topics of “race” and ethnicity (especially in political discussions) is actually counterproductive since the negative social consequences of modern “race” ideas could be ignored, making it harder to examine and address instances of discrimination properly (Wise 2010). Rather than shy away from these topics, we can use our scientific findings to establish socially relevant and biologically accurate ideas concerning human diversity. Today, research into genetic and phenotypic differentiation among and within various human populations continues to expand in its scope, its technological capabilities, its sample sizes, and its ethical concerns. It is thanks to such scientific work done in the past few decades that we now have a deeper understanding not only of how humans vary but also of how we are biologically a rather homogenous, intermixing world population.
SPECIAL TOPIC: MY EXPERIENCES AS A MINORITY ACADEMIC OF COLOR

My name is Michael, and I am a researcher in biological anthropology (Figure 13.20). What strikes me as most interesting to investigate is human biological diversity today and the study of past human evolution. What I am really curious about is how we can use human skeletons to infer how people adapt to coastal environments. Relying on aquatic foods near rivers, lakes, and the sea is interesting because we have found evidence for positive effects of coastal living on dietary health and many unique adaptations in bones and teeth when living near rivers and beaches. I also really enjoy talking to students and non-scientists about our work, through teaching, science communication events, and writing book chapters like this one! I grew up in Hong Kong, a city in southern China. My father is from the Philippines and my mother is from Hong Kong, which makes me a mixed Filipino-Chinese academic. When I attended international schools in my youth, I saw that kids my age came in all shapes, sizes, and colors. It was not until I left Hong Kong that I realized people with my skin tone were somewhat rarer in British universities I attended.
Biological anthropology is not taught extensively back home in Hong Kong, but my initial motivation to enter this field was a great TV show called Bones. This TV series was about a brilliant anthropologist who examined human remains for the Smithsonian Institute in Washington, D.C., identified the individuals they belonged to through scientific analyses of bones and teeth, and told the stories of the deceased who could not tell their own. I went to the United Kingdom to earn my bachelor’s, master’s, and doctorate degrees. During my studies, I was taught about human genetics, apes and monkeys, forensics, human cultural and behavioral diversity, and the story of human evolution that began six million to eight million years ago. It was fascinating to me that we could answer important questions about human variation and history using scientific methods. While I was at university, I did not have many minority academic role models to look up to. Today, I look around and see other academics of color during conferences and perhaps one or two others around the places at which I work. I am inspired by all my colleagues who advocate for greater representation and diversity in universities (whether they are minority academics or not). I admire many of my fellow researchers who are underrepresented and do a great job of representing minority groups through their cutting-edge research and quality teaching at the undergraduate and graduate levels. The study of anthropology has really highlighted for me that we share a common humanity and history. Being somebody who is “mixed race” and Asian likely played a key role in steering me toward a discipline that studies human diversity. As this chapter hopefully shows, there is a lot about race and ethnicity to discuss in terms of the discipline’s history and current understandings of human biological diversity. Some scientific and technological advancements today are unfortunately misused for reasons to do with money, politics, or the continuation of fairly antiquated ideas. It is my belief, alongside many of my friends and fellow anthropologists, that science should be more about empathy toward all members of our species and contributing to the intellectual and technological nourishment of society. After speaking to many members of the public, as well as my own undergraduate students, I have received lovely messages from other individuals of color expressing thanks and appreciation for my presence and understanding as a fellow minority and mentor figure. This is why anthropology needs more diversity and to make room for more personal routes into the discipline. All paths to anthropology are valuable and valid. I would encourage anyone to study anthropology as it really is a field for understanding and celebrating the intricacies of human diversity.
Review Questions
- How is the genetic variation of the human species distributed worldwide?
- What evolutionary processes are responsible for producing genotypic/phenotypic diversity within and between human populations?
- Should we continue to attribute any value in “race” concepts older than 1950, based on our current understandings of human biological diversity?
- How should we communicate scientific findings about human biological variation more accurately and responsibly to those outside the anthropological discipline?
Key Terms
Age of Discovery: A period between the late 1400s and late 1700s when European explorers and ships sailed extensively across the globe in pursuit of new trading routes and territorial conquest.
Ancestry: Biogeographical information about an individual, traced either through the study of an individual’s genome, skeletal characteristics, or some other form of forensic/archaeological evidence. Anthropologists carry out probabilistic estimates of ancestry. They attribute sets of human remains to distinctive “ancestral” groups using careful statistical testing and should report ancestry estimations with statistical probability values.
Binomial nomenclature: A system of naming living things developed by Linnaeus in the 1700s using a scientific name made up of two Latin- or Greek-form words, with the first name capitalized and representative of an organism’s genus and the second name indicating an organism’s species (e.g., Homo sapiens, Australopithecus afarensis, Pongo tapanuliensis, etc.).
Biological anthropology: A subdiscipline of anthropology concerned with the biological origins, ecology, evolution, and diversity of humans and other primates. This term is increasingly preferred to physical anthropology, as many in the field now uncomfortably associate this original name (coined by Aleš Hrdlička) with the ways in which questions of human variation were studied in decades past and the sociohistorical context that made anthropology problematic before 1950 (see Warren 2018).
Biological determinism: The erroneous concept that an individual’s behavioral characteristics are innate and determined by genes, brain size, or other physiological attributes, and with no influence of social learning or the environment around the individual during development.
Bony labyrinth: A system of interconnected canals within the auditory (ear- or hearing-related) apparatus, located in the inner ear and responsible for balance and the reception of sound waves.
Cline: A gradient of physiological or morphological change in a single character or allele frequency among a group of species across environmental or geographical lines (e.g., skin color varies clinally, as, over many generations, human groups living nearer the equator have adapted to have more skin pigmentation).
Continuous/clinal variation: Variation that exists between individuals and cannot be measured using distinct categories. Instead, differences between individuals within a population in relation to one particular trait are measurable along a smooth, continuous gradient.
Cystic fibrosis: A genetic disorder in which one defective gene causes overproduction and buildup of mucus in the lungs and other bodily organs, most common in northern Europeans (but also in other world populations more rarely).
Ecological niche: The position or status of an organism within its community and/or ecosystem, resulting from the organism’s structural and functional adaptations (e.g., bipedalism, omnivory, lactose digestion, etc.).
Essentialism: A belief or view that an entity, organism, or human grouping has a specific set of characteristics that are fundamentally necessary to its being and classification into definitive categories.
Ethnicity: A complex term used commonly in an interchangeable way with the term race (see below).
Eugenics: A set of beliefs and practices that involves the controlled selective breeding of human populations with the hope of improving their heritable qualities, especially through surgical procedures like sterilization and legal rulings that affect marriage rights for interracial couples.
Founder effect: See population bottlenecking below.
Gene flow: A neutral (or nonselective) evolutionary process that occurs when genes get shared between populations.
Genetic drift: A neutral evolutionary process in which allele frequencies from generation to generation due to random chance.
Heterogeneity: The quality of being diverse genetically.
Homogenous: The quality of being uniform genetically.
Human diversity/differentiation/variation: Group differences involving variation in biology, physiology, body chemistry, behavior, and culture.
Isolation-by-distance model: A model that predicts a positive relationship between genetic distances and geographical distances between pairs of populations.
Monogenic: Characterized as being controlled by a single gene (or, in other words, one pair of alleles). Sickle cell anemia and cystic fibrosis are examples of disorders that are monogenically caused.
Monogenetic: Pertaining to the idea that the origin of a species is situated in one geographic region or time (as opposed to polygenetic).
Mutation: A gene alteration in the DNA sequence of an organism. As a random, neutral evolutionary process that occurs over the course of meiosis and early cell development, gene mutations are possible sources of diversity in any given human gene pool. Genetic mutations that occur in more than 1% of a population are termed polymorphisms.
Natural selection: An evolutionary process whereby certain traits are perpetuated through successive generations, likely owing to the advantages they give organisms in terms of chances of survival and/or reproduction.
Non-concordance: The fact of genes or traits not varying with one another and instead being inherited independently.
Othering: In postcolonial anthropology, we now understand “othering” to mean any action by someone or some group that establishes a division between “us” and “them” in relation to other individuals or populations. This could be based on linguistic or cultural differences, and it has largely been based on external characteristics throughout history.
Out-of-Africa model: A model that suggests that all humans originate from one single group of Homo sapiens in (sub-Saharan) Africa who lived between 100,000 and 315,000 years ago and who subsequently diverged and migrated to other regions across the globe.
Physical anthropology: See biological anthropology above.
Polygenetic: Having many different ancestries, as in older theories about human origins that involved multiple traditional groupings of humans evolving concurrently in different parts of the world before they merged into one species through interbreeding and/or intergroup warfare. These earlier suggestions have now been overwhelmed by insurmountable evidence for a single origin of the human species in Africa (see the “Out-of-Africa model”).
Polymorphism: A genetic variant within a population (caused either by a single gene or multiple genes) that occurs at a rate of over 1% among the population. Polymorphisms are responsible for variation in phenotypic traits such as blood type and skin color.
Population: A group of humans living in a particular geographical area, with more local interbreeding within-group than interbreeding with other groups. A limited or restricted amount of gene flow between populations can occur due to geographical, cultural, linguistic, or environmental factors.
Population bottlenecking (or founder effect): An event in which genetic diversity is significantly reduced owing to a sharp reduction in population size. This can occur when environmental disaster strikes or as a result of human activities (e.g., genocides or group migrations). An important example of this loss in genetic variation occurred over the first human migrations out of Africa and into other continental regions.
Prejudice: An unjustified attitude toward an individual or group not based on reason, whether that is positive and showing preference for one group of people over another or negative and resulting in harm or injury to others.
Race: The identification of a group based on a perceived distinctiveness that makes that group more similar to each other than they are to others outside the group. This may be based on cultural differences, genetic parentage, physical characteristics, behavioral attributes, or something arbitrarily and socially constructed. As a social or demographic category, perceptions of “race” can produce effects that have real and serious consequences for different groups of people. This is despite the fact that biological anthropologists and geneticists have demonstrated that all humans are genetically homogenous and that more differences can be found within populations as opposed to between them in the overall apportionment of human biological variation.
Racism: Any action or belief that discriminates against someone based on perceived differences in race or ethnicity, and the characteristics, qualities, or abilities believed to be specific to a race that is inferior to another in some way.
Scientific Revolution: A period between the 1400s and 1600s when substantial shifts occurred in the social, technological, and philosophical sense, when a scientific method based on the collection of empirical evidence through experimentation was emphasized and inductive reasoning used to test hypotheses and interpret their results.
Typology: An assortment system that relies on the interpretation of qualitative similarities or differences in the study of variation among objects or people. The categorization of cultures or human groups according to “race” was performed with a typological approach in the earliest practice of anthropology, but this practice has since been discredited and abandoned.
About the Author
Michael B. C. Rivera
University of Cambridge, mbcr2@alumni.cam.ac.uk
The Arch and Anth Podcast, archandanthpodcast@gmail.com

Michael B. C. Rivera is a biological anthropologist and human bioarchaeologist, studying the transition into agriculture in coastal environments. His recently completed doctoral thesis brought together human skeletal biology, palaeopathology, and prehistoric archaeology to investigate the lives of ancient people on the northeastern European coastline. Being from Hong Kong and a student of human biological variation, Michael is also an advocate for greater inclusion, diversity, and equality in academia. Additionally, as a believer in the value of science communication, and of the value of the discipline to greater society, he launched The Arch and Anth Podcast in May 2019, which disseminates scientific knowledge in a fun, educational, and informal interview-style audio format.
References
Antrosio, Jason. 2011. “‘Race Reconciled’: Race Isn’t Skin Color, Biology, or Genetics.” Living Anthropologically Website. https://www.livinganthropologically.com/biological-anthropology/race-reconciled-debunks-race/.
Beals, Kenneth L., Courtland L. Smith, Stephen M. Dodd, J. Lawrence Angel, Este Armstrong, Bennett Blumenberg, Fakhry G. Girgis, et al. 1984. “Brain Size, Cranial Morphology, Climate, and Time Machines [and Comments and Reply].” Current Anthropology 25 (3): 301‒330.
Betti, Lia, François Balloux, William Amos, Tsunehiko Hanihara, and Andrea Manica. 2008. “Distance from Africa, Not Climate, Explains Within-Population Phenotypic Diversity in Humans.” Proceedings of the Royal Society B: Biological Sciences 276 (Issue 165B): 809‒814. doi:10.1098/rspb.2008.1563.
Betti, Lia, François Balloux, Tsunehiko Hanihara, and Andrea Manica. 2010. “The Relative Role of Drift and Selection in Shaping the Human Skull.” American Journal of Physical Anthropology 141 (1): 76‒82. doi:10.1002/ajpa.21115.
Betti, Lia, Noreen von Cramon-Taubadel, Andrea Manica, and Stephen J. Lycett. 2013. “Global Geometric Morphometric Analyses of the Human Pelvis Reveal Substantial Neutral Population History Effects, Even across Sexes.” PloS ONE 8 (2): e55909. doi:10.1371/journal.pone.0055909.
———. 2014. “The Interaction of Neutral Evolutionary Processes with Climatically Driven Adaptive Changes in the 3D Shape of the Human Os Coxae.” Journal of Human Evolution 73 (August): 64‒74. doi:10.1016/j.jhevol.2014.02.021.
Boas, Franz. 1931. “Race and Progress.” Science 74 (1905): 1‒8.
Bowden, Rory, Tammie S. MacFie, Simon Myers, Garrett Hellenthal, Eric Nerrienet, Ronald E. Bontrop, Colin Freeman, Peter Donnelly, and Nicholas I. Mundy. 2012. “Genomic Tools for Evolution and Conservation in the Chimpanzee: Pan troglodytes ellioti Is a Genetically Distinct Population.” PLoS Genetics 8 (3): e1002504. doi:10.1371/journal.pgen.1002504.
Gerbault, Pascale, Anke Liebert, Yuval Itan, Adam Powell, Mathias Currat, Joachim Burger, Dallas M. Swallow, and Mark G. Thomas. 2011. “Evolution of Lactase Persistence: An Example of Human Niche Construction.” Philosophical Transactions of the Royal Society B 366 (1566): 863‒877. doi:10.1098/rstb.2010.0268.
Hooton, Earnest A. 1936. “Plain Statements about Race.” Science 83 (2161): 511‒513.
Hrdlička, Aleš. 1918. “Physical Anthropology: Its Scope and Aims; Its History and Present Status in America. A: Physical Anthropology; Its Scopes and Aims.” American Journal of Physical Anthropology 1 (1): 3‒23.
Huxley, Julian. 1942. Evolution: The Modern Synthesis. London: Allen and Unwin.
Ingram, Catherine J. E., Charlotte A. Mulcare, Yuval Itan, Mark G. Thomas, and Dallas M. Swallow. 2009. “Lactose Digestion and the Evolutionary Genetics of Lactase Persistence.” Human Genetics 124 (6): 579‒591. doi:10.1007/s00439-008-0593-6.
Jablonski, Nina G. 2004. “The Evolution of Human Skin and Skin Color.” Annual Review of Anthropology 33 (1): 585‒623. doi:10.1146/annurev.anthro.33.070203.143955.
Jablonski, Nina G., and George Chaplin. 2000. “The Evolution of Human Skin Coloration.” Journal of Human Evolution 39 (1): 57‒106. doi:10.1006/jhev.2000.0403.
Kronenberg, Zev N., Ian T. Fiddes, David Gordon, Shwetha Murali, Stuart Cantsilieris, Olivia S. Meyerson, Jason G. Underwood, et al. 2018. “High-Resolution Comparative Analysis of Great Ape Genomes.” Science 360 (6393): eaar6343. doi:10.1126/science.aar6343.
Lewontin, Richard. 1972. “The Apportionment of Human Diversity.” Evolutionary Biology 6: 381‒398. Eds. Dobzhansky, Theodosius, Hecht, Max K., Steere, William C. Springer, New York, NY
Linnaeus, Carl. 1758. Systema Naturae. http://www.cabdirect.org/abstracts/20057000018.html.
Liu, Hua, Franck Prugnolle, Andrea Manica, and François Balloux. 2006. “A Geographically Explicit Genetic Model of Worldwide Human-Settlement History.” American Journal of Human Genetics 79 (2): 230‒237.
Livingstone, Frank B. 1962. “On the Nonexistence of Human Races.” Current Anthropology 3 (3): 279‒281.
Long, Jeffery C., and Kittles, Rick A. 2003. “Human Genetic Diversity and the Nonexistence of Biological Races.” Human Biology 75 (4): 449‒471.
Luzzatto, Lucio. 2012. “Sickle Cell Anaemia and Malaria.” Mediterranean Journal of Hematology and Infectious Diseases 4 (1). doi:10.4084/MJHID.2012.065.
Manica, Andrea, William Amos, François Balloux, and Tsunehiko Hanihara. 2007. “The Effect of Ancient Population Bottlenecks on Human Phenotypic Variation.” Nature 448 (7151): 346‒348. doi:10.1038/nature05951.
Mourant, A. E., Ada C. Kopeć, and Kazimiera Domaniewska-Sobczak. 1976. The Distribution of the Human Blood Groups and Other Polymorphisms. 2nd edition. Oxford: Oxford University Press.
Morton, Samuel George. 1839.Crania Americana, or, A comparative view of the skulls of various aboriginal nations of North and South America. Philadelphia: J. Dobson.
National Research Council (U.S.) Committee on Human Genome Diversity. 1997. Evaluating Human Genetic Diversity. Washington, D.C.: National Academies Press
Ousley, Stephen D., Richard L. Jantz, and Donna Freid. 2009. “Understanding Race and Human Variation: Why Forensic Anthropologists Are Good at Identifying Race.” American Journal of Physical Anthropology 139 (1): 68‒76. doi:10.1002/ajpa.21006.
Oxford English Dictionary. 2018. 3rd edition. Oxford: Oxford University Press.
Ponce de León, Marcia S., Toetik Koesbardiati, John David Weissmann, Marco Millela, Carlos S. Reyna-Blanco, Gen Suwa, Osamu Kondo, Anna-Sapfo Malaspinas, Tim D. White, and Christoph P. E. Zollikofer. 2018. “Human Bony Labyrinth Is an Indicator of Population History and Dispersal from Africa.” Proceedings of the National Academy of Sciences 115 (16): 4128‒4133. doi:10.1073/pnas.1808125115.
Prado-Martinez, Javier, Peter H. Sudmant, Jeffrey M. Kidd, Heng Li, Joanna L Kelley, Belen Lorente-Galdos, Krishna R. Veeramah, et al. 2013. “Great Ape Genetic Diversity and Population History.” Nature 499 (7459): 471–475. doi:10.1038/nature12228.
Prugnolle, Franck, Andrea Manica, and François Balloux. 2005. “Geography Predicts Neutral Genetic Diversity of Human Populations.” Current Biology 15 (5): 159‒160.
Rathmann, Hannes, Hugo Reyes-Centeno, Silvia Ghirotto, Nicole Creanza, Tsunehiko Hanihara, and Katerina Harvati. 2017. “Reconstructing Human Population History from Dental Phenotypes.” Scientific Reports 7: 12495. doi:10.1038/s41598-017-12621-y.
Relethford, John H. 2001. “Global Analysis of Regional Differences in Craniometric Diversity and Population Substructure.” Human Biology 73 (5): 629‒636. doi:10.1353/hub.2001.0073.
———. 2002. “Apportionment of Global Human Genetic Diversity Based on Craniometrics and Skin Color.” American Journal of Physical Anthropology 118 (4): 393‒398. doi:10.1002/ajpa.10079.
———. 2004. “Global Patterns of Isolation by Distance Based on Genetic and Morphological Data.” Human Biology 76 (4): 499‒513. doi:10.1353/hub.2004.0060.
———. 2009. “Race and Global Patterns of Phenotypic Variation.” American Journal of Physical Anthropology 139 (1): 16‒22. doi:10.1002/ajpa.20900.
Rosenberg, Noah A., Saurabh Mahajan, Sohini Ramachandran, Chengfeng Zhao, Jonathan K. Pritchard, and Marcus W. Feldman. 2005. “Clines, Clusters, and the Effect of Study Design on the Inference of Human Population Structure.” PLoS Genetics 1 (6): e70. doi:10.1371 /journal.pgen.0010070.
Rosenberg, Noah A., Jonathan K. Pritchard, James L. Weber, Howard M. Cann, Kenneth K. Kidd, Lev A. Zhivotovsky, and Marcus W. Feldman. 2002. “Genetic Structure of Human Populations.” Science 298 (5602): 2381‒2385.
Sauer, Norman J. 1992. “Forensic Anthropology and the Concept of Race: If Races Don’t Exist, Why Are Forensic Anthropologists So Good at Identifying Them?” Social Science and Medicine 34 (2): 107‒111. doi:10.1016/0277-9536(92)90086-6.
Staes, Nicky, Chet C. Sherwood, Katharine Wright, Marc de Manuel, Elaine E. Guevara, Tomas Marques-Bonet, Michael Krützen, et al. 2017. “FOXP2 Variation in Great Ape Populations Offers Insight into the Evolution of Communication Skills.” Scientific Reports 7 (1): 1‒10. doi:10.1038/s41598-017-16844-x.
von Cramon-Taubadel, Noreen, and Stephen J. Lycett. 2008. “Brief Communication: Human Cranial Variation Fits Iterative Founder Effect Model with African Origin.” American Journal of Physical Anthropology 136 (1): 108‒113. doi:10.1002/ajpa.20775.
Warren, Kerryn A. 2018. “AAPA Name Change.” Bone and Evolution, April 4. https://bonevolution.wordpress.com/2018/04/04/aapa-name-change/.
Weiss, Kenneth M., and Jeffrey C. Long. 2009. “Non-Darwinian Estimation: My Ancestors, My Genes’ Ancestors.” Genome Research 19: 703‒710. doi:10.1101/gr.076539.108.19.
Wise, Tim. 2010. Colorblind: The Rise of Post-Racial Politics and the Retreat from Racial Equity. San Francisco: City Lights.
Yudell, Michael, Dorothy Roberts, Rob DeSalle, and Sarah Tishkoff. 2016. “Taking Race out of Human Genetics.” Science 351 (6273): 564‒565. doi:10.1126/science.aac4951.
Figure Attributions
Figure 13.1a Tanzania – Hadzabe hunter (14533536392) by A_Peach from Berlin, Germany, is used under a CC BY 2.0 License.
Figure 13.1b Inuit-Kleidung 1 by Ansgar Walk is used under a CC BY-SA 3.0 License.
Figure 13.1c Andean Man by Cacophony is used under a CC BY-SA 4.0 License.
Figure 13.1d Jane Goodall GM byFloatjon is used under a CC BY-SA 3.0 License.
Figure 13.2 Egyptian races Drawing (1772-1846) by an unknown artist after a mural of the tomb of Seti I, Copy by Heinrich von Minutoli (1820), is in the public domain.
Figure 13.3 Naturalishistoria from the front page of Pliny the Elder’s Naturalis Historia is in the public domain.
Figure 13.4 Great Chain of Being 2 by Didacus Valades (Diego Valades) is in the public domain.
Figure 13.5 Carl von Linné by Alexander Roslin artist QS:P170,Q315102 is in the public domain.
Figure 13.6 Discovery of the Mississippi by William Henry Powell artist QS:P170,Q3568696 (photograph courtesy Architect of the Capitol) is in the public domain.
Figure 13.7 Blumenbach’s five races by Johann Friedrich Blumenbach is in the public domain.
Figure 13.8 (Ales Hrdlicka) SIA2009-4246 by Unknown photographer is in the public domain.
Figure 13.9 Eugenics congress logo scanned from Harry H. Laughlin, The Second International Exhibition of Eugenics held September 22 to October 22, 1921, is in the public domain.
Figure 13.10 Dobzhansky no Brasil em 1943 by Unknown photographer is in the public domain.
Figure 13.11 Julian Huxley 1-2 by Unknown photographer is in the public domain.
Figure 13.12 Skin color by S25454541 is used under a CC BY-SA 4.0 License.
Figure 13.13a Map of blood group a by Muntuwandi at en.wikipedia is used under a CC BY-SA 3.0 License.
Figure 13.13b Map of blood group b by Muntuwandi at en.wikipedia is used under a CC BY-SA 3.0 License.
Figure 13.13c Map of blood group o Based on diagrams from http://anthro.palomar.edu/vary/vary_3.htm reproduced from A. E. Mourant et.al., The Distribution of the Human Blood Groups and Other Polymorphisms, 2nd ed. (1976) is used under a CC BY-SA 3.0 License.
Figure 13.14 Sub-Saharan-Africa by Ezeu has been designated to the public domain (CC0).
Figure 13.15 Bottleneck effect by Tsaneda is used under a CC BY 3.0 License.
Figure 13.16 Chimpanzee IV (13968482163) by Chi King is used under a CC BY 2.0 License.
Figure 13.17 Human skulls by 22Kartika is used under a CC BY-SA 3.0 License.
Figure 13.18 Bony labyrinth by Selket (5 February 2007, UTC) has been designated to the public domain (CC0).
Figure 13.19 Forensic Anthropology Lab by Pp391 is used under a CC BY-SA 3.0 License.
Figure 13.20 Michael B. C. Rivera in Hong Kong original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
A subdiscipline of anthropology concerned with the biological origins, ecology, evolution, and diversity of humans and other primates. This term is increasingly preferred to physical anthropology.
Group differences involving variation in biology, physiology, body chemistry, behavior, and culture.
The identification of a group based on a perceived distinctiveness that makes that group more similar to each other than they are to others outside the group. This may be based on cultural differences, genetic parentage, physical characteristics, behavioral attributes, or something arbitrarily and socially constructed. As a social or demographic category, perceptions of “race” can produce effects that have real and serious consequences for different groups of people. This is despite the fact that biological anthropologists and geneticists have demonstrated that all humans are genetically homogenous and that more differences can be found within populations as opposed to between them in the overall apportionment of human biological variation.
A complex term used commonly in an interchangeable way with the term race (see below).
Any action or belief that discriminates against someone based on perceived differences in race or ethnicity, and the characteristics, qualities, or abilities believed to be specific to a race that is inferior to another in some way.
An unjustified attitude toward an individual or group not based on reason, whether that is positive and showing preference for one group of people over another or negative and resulting in harm or injury to others.
The quality of being uniform genetically.
A period between the 1400s and 1600s when substantial shifts occurred in the social, technological, and philosophical sense, when a scientific method based on the collection of empirical evidence through experimentation was emphasized and inductive reasoning used to test hypotheses and interpret their results.
A system of naming living things developed by Linnaeus in the 1700s using a scientific name made up of two Latin- or Greek-form words, with the first name capitalized and representative of an organism’s genus and the second name indicating an organism’s species (e.g., Homo sapiens, Australopithecus afarensis, Pongo tapanuliensis, etc.).
A belief or view that an entity, organism, or human grouping has a specific set of characteristics that are fundamentally necessary to its being and classification into definitive categories.
A period between the late 1400s and late 1700s when European explorers and ships sailed extensively across the globe in pursuit of new trading routes and territorial conquest.
In postcolonial anthropology, we now understand “othering” to mean any action by someone or some group that establishes a division between “us” and “them” in relation to other individuals or populations. This could be based on linguistic or cultural differences, and it has largely been based on external characteristics throughout history.
The idea that different peoples have different origins.
Characterized as being controlled by a single gene (or, in other words, one pair of alleles). Sickle cell anemia and cystic fibrosis are examples of disorders that are monogenically caused.
The erroneous concept that an individual’s behavioral characteristics are innate and determined by genes, brain size, or other physiological attributes, without the influence of social learning or the environment around the individual during development.
See biological anthropology.
An idea that was popular in the 1920s that society should be improved by breeding better kinds of people.
An assortment system that relies on the interpretation of qualitative similarities or differences in the study of variation among objects or people. The categorization of cultures or human groups according to “race” was performed with a typological approach in the earliest practice of anthropology, but this practice has since been discredited and abandoned.
A group of individuals who are genetically similar enough and geographically near enough to one another that they can breed and produce new generations of individuals.
A gradient of physiological or morphological change in a single character or allele frequency among a group of species across environmental or geographical lines (e.g., skin color varies clinally, as, over many generations, human groups living nearer the equator have adapted to have more skin pigmentation).
Variation that exists between individuals and cannot be measured using distinct categories. Instead, differences between individuals within a population in relation to one particular trait are measurable along a smooth, continuous gradient.
An evolutionary process that occurs when certain phenotypes confer an advantage or disadvantage in survival and/or reproductive success. This is one of the forces of evolution.
The movement of alleles from one population to another. This is one of the forces of evolution.
Random changes in allele frequencies within a population from one generation to the next. This is one of the forces of evolution.
A type of genetic drift that occurs when members of a population leave the main or “parent” group and form a new population that no longer interbreeds with the other members of the original group.
Theory that modern Homo sapiens expanded from Africa to cover the rest of the world without interacting with archaic humans.
A model that predicts a positive relationship between genetic distances and geographical distances between pairs of populations.
A type of genetic drift that occurs when the number of individuals in a population drops dramatically due to some random event.
A change in the nucleotide sequence of the genetic code. This is one of the forces of evolution.
The quality of being diverse genetically.
The position or status of an organism within its community and/or ecosystem, resulting from the organism’s structural and functional adaptations (e.g., bipedalism, omnivory, lactose digestion, etc.).
The fact of genes or traits not varying with one another and instead being inherited independently.
A system of interconnected canals within the auditory (ear- or hearing-related) apparatus, located in the inner ear and responsible for balance and the reception of sound waves.
A genetic disorder in which one defective gene causes overproduction and buildup of mucus in the lungs and other bodily organs, most common in northern Europeans (but also in other world populations more rarely).
Biogeographical information about an individual, traced either through the study of an individual’s genome, skeletal characteristics, or some other form of forensic/archaeological evidence. Anthropologists carry out probabilistic estimates of ancestry. They attribute sets of human remains to distinctive “ancestral” groups using careful statistical testing and should report ancestry estimations with statistical probability values.
Multiple forms of a trait; alternative phenotypes within a given species.
