Private: Appendices
18 Primate Conservation
Mary P. Dinsmore, M.S., Ph.D. candidate, University of Wisconsin–Madison
Ilianna E. Anise, B.A., Ph.D. student, University of Wisconsin–Madison
Rebekah J. Ellis, B.A., M.S., University of Wisconsin–Madison
Amanda J. Hardie, M.A., Ph.D. candidate, University of Wisconsin–Madison
Jacob B. Kraus, B.A., Ph.D. student, University of Wisconsin–Madison
Karen B. Strier, Ph.D., University of Wisconsin–Madison
Learning Objectives
- Understand the current conservation status of the world’s primates and the criteria that researchers and conservationists use to make these assessments.
- Recognize the many threats that negatively impact primate survival.
- Understand how these threats uniquely affect primates because of characteristics like slow growth rates, long interbirth intervals, strong social bonds, and cultural behavior.
- Become aware of the many ways in which primates are significant to ecological processes, our understanding of human evolution, human cultures, and local economies.
- Learn about ways that people, wherever they may live, can work to protect primates.
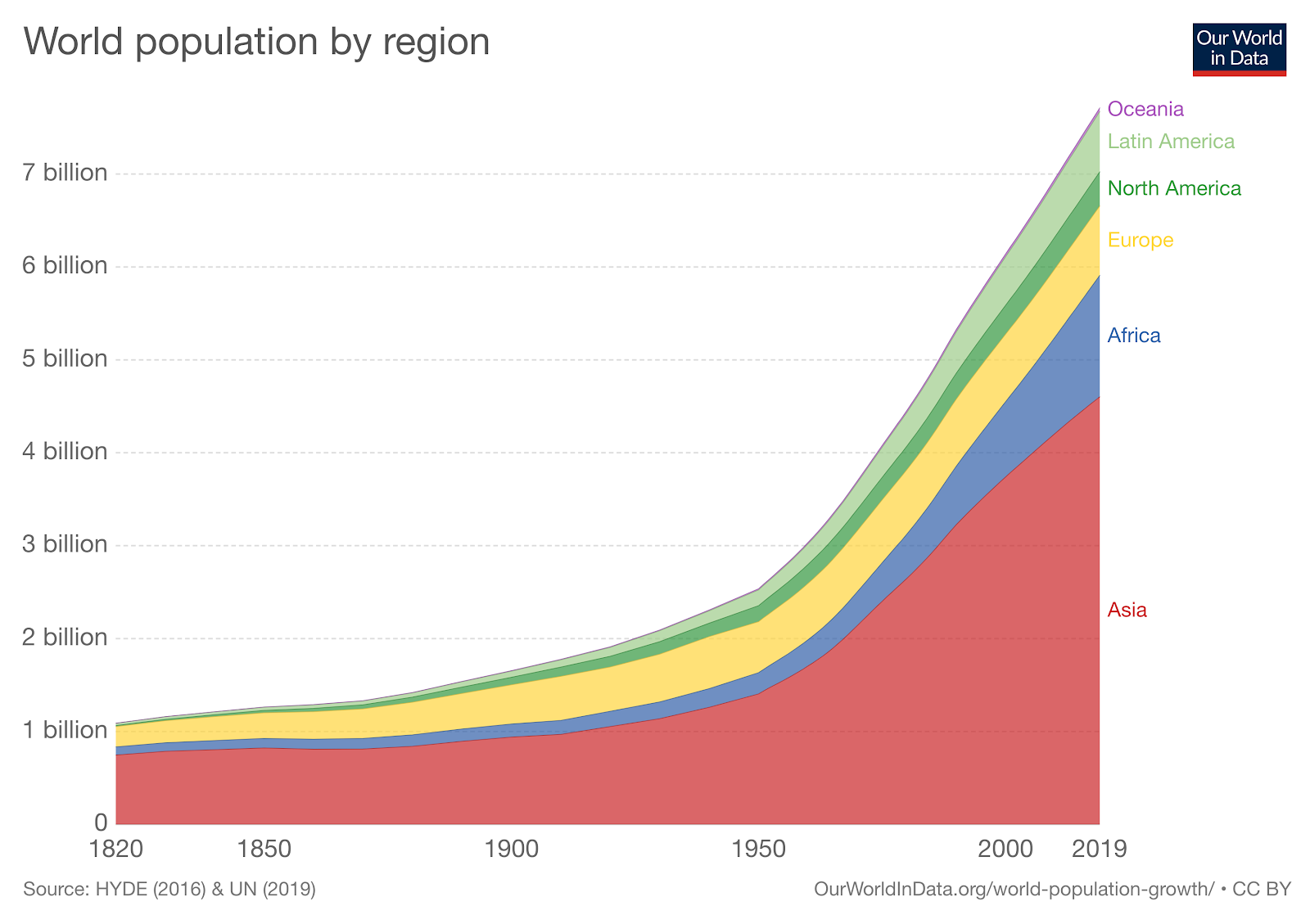
We are field primatologists interested in understanding primates in their natural environments and in contributing to their conservation. Our research focuses on a diversity of primate species that occur in a wide range of habitats throughout the tropics; however, these species and their habitats are subject to many similar threats. As human populations continue to grow (Figure B.1), primates are being pushed out of their natural home ranges and are being forced to occupy increasingly smaller and more isolated patches of land. Humans and primates are sharing more spaces with one another, making it easier for primates to be hunted or captured and for diseases to spread from humans to primates (and vice versa). Even when primates are not directly threatened by human activities, human-induced climate change is altering local ecosystems at an alarming rate. Local political instability exacerbates all of these problems. Our research causes us to think about these issues on a daily basis. Understanding how these threats affect the primates we study is a very important part of what we do. Ultimately, the research of field primatologists like us is important for documenting the status of wild primate populations, as well as for understanding how they respond to these threats and for gaining insights into the kinds of efforts that can help to improve their chances of survival in an uncertain future.
This appendix begins with a review of the current status of primates and the criteria used in these assessments. We then describe the major threats to primates, explain why primates are important, and consider what can be done to improve their chances. We conclude with a brief consideration of the future for primates.
CURRENT CONSERVATION STATUS OF NONHUMAN PRIMATES
Diversity of Primates
The order Primates is one of the most diverse groups of mammals on the planet, with 504 species in 79 different genera currently recognized (Figure B.2; Estrada et al. 2017). Recently new genera, species, and subspecies of nonhuman primates (henceforth, simply “primates” ) have been recognized, in some cases as a result of new discoveries and new data, but also because of revisions to taxonomic classification systems based on different species concepts (Groves 2014; Lynch Alfaro et al. 2012; Rylands and Mittermeier 2014).



Wild primates occur in 90 countries around the world, but two-thirds of all species are found in only four countries: Brazil, Madagascar, Democratic Republic of Congo, and Indonesia (Estrada et al. 2017; Estrada et al. 2018). An estimated 60% of primate species are threatened with extinction and 75% are experiencing population declines (Estrada et al. 2017, see Figure B.2). Yet despite these discouraging statistics, there are a growing number of populations recovering as a result of research and conservation efforts. For example, the population of mountain gorillas (Figure B.3) initially studied by Dian Fossey in Rwanda in 1967 has increased from 250 gorillas in 1981 to 339 in 2008 as a result of ongoing research and conservation efforts that include highly controlled ecotourism (Robbins et al. 2011). Similarly, one population of northern muriqui monkeys (Figure B.4) inhabiting a small privately owned forest fragment in southeastern Brazil’s Atlantic Forest increased from about 50 individuals to nearly 350 individuals as a result of increased habitat protection over the course of the Muriqui Project of Caratinga, a long-term field study initiated more than 35 years ago by one of the authors of this appendix (Strier and Mendes 2012).
International Union for the Conservation of Nature (IUCN)
In conservation, it is crucial to have a global standard to assess and recognize the conservation status of species. The International Union for the Conservation of Nature (IUCN) formed the Red List for Threatened Species in 1994 to determine species extinction risks (IUCN 2017). Scientists submit assessments of species to the IUCN, which are subsequently categorized based on the size and distribution of species’ numbers and available habitat. The categories range from “data deficient,” when not enough is known, to “least concern,” “near threatened,” “vulnerable,” “endangered,” “critically endangered,” “extinct in the wild,” and “extinct.” Threatened species are classified as “vulnerable,” “endangered,” or “critically endangered,” with the most critically endangered species being those whose numbers are fewer than 250 mature individuals and continuing to decline or whose habitats are severely fragmented (Figure B.5; IUCN 2017).
| Critically Endangered (CR): Facing an extremely high risk of extinction in the wild due to any of the following:
A. Reduction in population size of 80%–90% over the last ten years or three generations, depending on the causes and reversibility of the reductions; B. Extent of occurrence <100 km2 or area of occupancy <10 km2 or both; C. Population size estimated to number fewer than 250 mature individuals and to be declining or unevenly distributed; D. Population size estimated to number fewer than 50 mature individuals; E. Probability of extinction within ten years or three generations is at least 50%.
Endangered (EN): Facing a very high risk of extinction in the wild due to any of the following: A. Reduction in population size of 50%–70% over the last ten years or three generations, depending on the causes and reversibility of the reductions; B. Extent of occurrence <5000 km2 or area of occupancy <500 km2 or both; C. Population size estimated to number fewer than 2,500 mature individuals and to be declining or unevenly distributed; D. Population size estimated to number fewer than 250 mature individuals; E. Probability of extinction within 20 years or five generations is at least 20%.
Vulnerable (VU): Facing a high risk of extinction in the wild due to any of the following: A. Reduction in population size of 30%–50% over the last ten years or three generations, depending on the causes and reversibility of the reductions; B. Extent of occurrence <20,000 km2 or area of occupancy <2000 km2 or both; C. Population size estimated to number fewer than 10,000 mature individuals and to be declining or unevenly distributed; D. Population size estimated to number fewer than 1,000 mature individuals; E. Probability of extinction within 100 years is at least 10%. |
Figure B.5 International Union for Conservation of Nature (IUCN) Criteria for Threatened Taxa. Updated from Strier 2011a. Source: Simplified and condensed from IUCN Species Survival Commission, 2012.
The IUCN has a committee specifically dedicated to primates, the IUCN Species Survival Commission (SSC) Primate Specialist Group. This group collaborates with the International Primatological Society (IPS), Conservation International (CI), and the Bristol Zoological Society (BZS) every two years to publish “Primates in Peril: The World’s 25 Most Endangered Primates.” These lists are created at IPS open meetings and are intended to focus attention on all endangered primates by highlighting the plights of some of the most critically endangered (Schwitzer et al. 2017).
Identifying Priorities in Primate Conservation
It is important to consider extinction risk in making conservation decisions, thus the IUCN Red list and the “Primates in Peril” reports are factors in deciding how to allocate resources and funding. Some primate species are found only in biodiversity hot spots, or areas that contain high levels of species diversity and include primates that are endemic to the area and genetically unique (Sechrest et al. 2002). Hot spots are often considered conservation priorities because protecting these areas can result in the protection of large numbers of species. In addition, some conservation organizations focus on highly charismatic primate species (e.g., primates that are large, closely related to humans, or well-known from zoos, such as the golden lion tamarin) to garner attention and resources for conservation (Figure B.6). However, dramatic declines of charismatic species indicate that charisma is not enough (Estrada et al. 2017). For example, it is estimated that the population of Bornean orangutans (Pongo pygmaeus) decreased by 100,000 individuals between 1999 and 2015, despite being very popular with the general public (Voigt et al. 2018). In making conservation decisions, primatologists may also consider the importance of genetically unique primates, such as the aye-aye (Daubentonia madagascariensis), the last remaining species within its genus in order to preserve evolutionary history (Strier 2011a).
THREATS TO PRIMATES
Hunting, Poaching, and Wildlife Trade

Hunting represents one of the most critical threats to primates (Figure B.7). Bushmeat, which is the meat of wild animals, has historically been a staple diet in many societies. However, human population growth and economic development have increased the commercialization of bushmeat hunting, thereby increasing its impact (Estrada et al. 2017). The increased availability and use of shotguns has also dramatically increased the volume of carcasses that hunters capture (Cronin et al. 2015). Across 89 markets in Nigeria and Cameroon, John Fa and colleagues (2015) calculated that almost 150,000 primate carcasses (from at least 16 different species) were sold annually. In one market on the Liberia/Ivory Coast border, Ryan Covey and Scott McGraw (2014) estimated that the carcasses of nearly 9,500 primates (from at least nine different species) were sold per year, resulting in an almost 3% annual reduction in the local primate population.
Not all primates are hunted specifically for food. Biomedical researchers use primates as models for understanding human biology and as test subjects for the development of vaccines, drugs, and hormones (Conaway 2011). Many of these experiments require large numbers of primates; therefore biomedical facilities often require a continuous supply of primates. To support the international demand for biomedical research test subjects, rhesus macaques (Macaca mulatta) in northern and central India experienced a 90% decline in populations over a 28-year period, from 1959 to 1986 (Southwick and Siddiqi 1988). Between 2007 and 2008, a single biomedical laboratory purchased roughly 4,000 nocturnal monkeys for over 100,000 USD through a network of 43 traders across Brazil, Colombia, and Peru (Maldonado et al. 2009).

Aside from biomedical research, captured primates are both legally and illegally sold to pet owners, zoos, tourist centers, and circuses. In Peru, it is estimated that, as recently as 2015, hundreds of thousands of primates are illegally traded every year, comparable to levels of trade prior to a 1973 national ban on primate exportation (Shanee et al. 2017). Once captured, primates may spend over a week in transit from a rural village to a coastal market. To make the transportation of primates more manageable, common trafficking strategies include sedation, asphyxiation, electrocution, and the removal of teeth. As these conditions severely affect the health of the trafficked primates, many perish during the journey while others die within the hands of authorities. Out of the 77 greater slow lorises (Nycticebus coucang) confiscated from a single wildlife trader in Indonesia, 22 died from either trauma or from the severity of their wounds (Fuller et al. 2018). Even when primates are successfully confiscated from wildlife traders, it is not uncommon for authorities to either resell or gift these animals to friends and family (Shanee et al. 2017).
To help curb illegal trafficking of animals, the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) was established in 1973 and ratified in 1975. Under this treaty, the 183 participating countries work together to both regulate the international trade of wildlife and to prevent the overexploitation of wild populations. While only some primates are listed as endangered or threatened under the Endangered Species Act (ESA), all primates are listed under CITES. According to the CITES database, more than 450,000 live primates were traded over the past 15 years (CITES n.d.). However, as the CITES database only includes information formally reported by each country, the real number of primates involved is likely to be much higher.
Habitat Loss, Fragmentation, and Degradation
The geographic distribution of many primate species has been severely limited by habitat loss. A recent analysis showed human demands for agricultural land threaten 76% of primate species, followed by demands for logging (60%) and livestock farming (31%) (Estrada et al. 2017). Habitat loss is not new and has affected the distribution of some primate species for thousands of years. Since the Bronze Age, for example, increases in human land use throughout China have been associated with corresponding decreases in suitable habitats for golden snub-nosed monkeys (Rhinopithecus roxellana) (Wang et al. 2014). However, our ever-growing need for food, water, and other natural resources has drastically decreased primate habitats globally (Figure B.8). From 2000 to 2013, roughly 220,000 km2 of tropical forest have been completely deforested in the Brazilian Amazon alone (Tyukavina et al. 2017). Since the start of oil palm development in Indonesia’s Ketapang District in 1994, over 65% of habitats without government protection have been allocated to the oil palm industry (Carlson et al. 2012). Even within protected areas, primate habitats are rapidly declining. In South Asia, 36% of surveyed protected areas had more than half of their habitat modified for human use, many of which experienced near-total habitat transformation (Clark et al. 2013). One of these protected areas, the Borajan Reserve Forest in India, saw a more than two-thirds reduction in suitable habitat within a three-year period, resulting in severe population declines for five primate species (Srivastava et al. 2001).

Habitat fragmentation compounds the effects of habitat loss. Whereas habitat loss reduces the total area in which primates can survive, habitat fragmentation divides large, contiguous primate habitats into smaller isolated patches (Figure B.9). The construction of road networks cutting through savannas, forests, and other primate habitats is a key driver of this fragmentation. Within the next half-century, over 25,000,000 kmof new roads are expected to be built, many of which in developing nations through primate habitats (Laurance et al. 2014). By fragmenting habitats, it becomes increasingly challenging for primates (particularly arboreal primates) to disperse between isolated habitat patches. While only 0.1% of black-and-white snub-nosed monkey (Rhinopithecus bieti) habitat was lost to the construction of China National Highway 214, movement between habitat patches on either side of the highway was reduced by over 20% (Clauzel et al. 2015). In the long run, habitat fragmentation can force primate populations into genetic bottlenecks, which occur when populations become so small that genetic diversity in them is severely reduced. In the forest fragments of Manaus, Brazil, groups of pied tamarins (Sanguinus bicolor) that historically formed one biological population were found to harbor only a subset of the genetic diversity previously exhibited in the region (Farias et al. 2015). Furthermore, primates living in fragments with scarce resources experience elevated levels of stress, which can also have long-term consequences on the health of individuals and populations (Rimbach et al. 2014).


Aside from habitat loss, other drivers of habitat degradation may affect primate populations. For example, streams can carry toxic chemicals used for agriculture into local habitats where they are either directly or indirectly consumed by primates. In Uganda, chimpanzees (Pan troglodytes) living within the Sebitoli Forest have been spotted with facial and limb deformities that are suspected of being related to their exposure to pesticides and herbicides used by local tea farmers (Krief et al. 2017). Additionally, invasive species that outcompete native species and alter habitats can affect primate behaviors. In Madagascar, southern bamboo lemurs (Hapalemur meridionalis) spent less time feeding in forests dominated by invasive Melaleuca trees (Melaleuca quinquenervia) than in forests without these trees (Eppley et al. 2015). Lastly, the use of fuelwood and charcoal is still widely used throughout sub-Saharan Africa as a means to produce heat and energy for cooking (Figure B.10).
Climate Change
Current State
Climate change is one of the most devastating threats facing primates because it compounds preexisting threats, such as habitat degradation and fragmentation. In a little over a century, the earth has seen temperatures rise by 0.85ºC globally, with each decade being warmer than the last (IPCC Climate Change 2014). The resulting changes that occur, many of which are just beginning to be documented, can be unpredictable and cause a range of consequences for biodiversity. Increasingly frequent regional droughts, especially in the Southern Hemisphere, not only affect resource abundance but also create a cascade of other ecosystem disturbances that contribute to increased carbon dioxide production (Zhao and Running 2010). In Kibale National Park, Uganda, Jessica Rothman and colleagues (2015) found that the increasing carbon dioxide levels associated with climate change decreased the nutritional value of leaves, an important food resource for many primates.
Climate change is associated with volatile and unpredictable weather patterns. El Niño Southern Oscillation events (ENSO) influence weather patterns across the globe. Although ENSO events have been occurring for thousands of years, climate change is causing them to occur more frequently and with greater intensity (Wiederholt and Post 2010). The warm air and unpredictable rains that come with an ENSO event can negatively affect food resource abundance. Primates whose diets include high quantities of fruit experience the effects of fruit shortages most strongly (Campos et al. 2015). Madagascar, a primate hot spot and country with high levels of anthropogenic disturbance, is a prime example of how the increased intensity and frequency of climatic events such as ENSO can have consequences for primates (Figure B.11). Amy Dunham and colleagues (2008) found that even the folivorous Milne-Edwards’ sifaka (Propithecus edwarsi), found within Ranomafana National Park in southeastern Madagascar, would experience severely reduced populations within three generations if ENSO events continued at the current frequency.

Rapidly changing climate also causes other extreme weather events in primate areas. Due to climate change, hurricanes and cyclones are occurring more frequently. Several studies have looked at the effects on primate populations before and after hurricanes (e.g., Ratsimbazafy 2006; Schaffner et al. 2012; Zimmerman and Kovich 2007). A population of howler monkeys in Belize (Alouatta pigra) experienced population reduction and reduced reproduction after a hurricane hit. This response lasted more than three years (Behie and Pavelka 2005; Pavelka and Chapman 2006). However, not all primates are as vulnerable to the effects of major storms. In Kirindy Mitea National Park in western Madagascar, Verreaux’s sifakas (Propithecus verreauxi) did not suffer reduced population or reproduction, despite a reduction in their food supply following a cyclone (Lewis and Rakontondranaivo 2011). The different responses of these species to catastrophic events is likely related to differences in their behavioral flexibility, such as adjusting the types of foods consumed and the amount of time spent resting, feeding, moving, and socializing by each species (Strier 2017) and evolutionary adaptability (Wright 1999).
Large-Scale Change
On a large scale, the deleterious effects of climate change can make primates’ current environments inhospitable. Most primates are adapted to live in tropical environments and many have specialized diets. Climate change alters the flowering and fruiting seasons of many plants, requiring a great deal of dietary flexibility from the organisms that rely on their production (Anderson et al. 2012). Many primates are not capable of this adjustment and would need to shift their habitat range to cope. Unfortunately, habitat loss and fragmentation make these range shifts impossible for many species without human assistance in the form of translocations. Primates have relatively slow life-histories, often producing only one offspring at a time, and their extended juvenile period results in slow evolutionary adaptation to change (Campos et al. 2017). To cope with the large-scale effects of climate change, primates must rely on behavioral flexibility and aid from humans. Primates are projected to have some of the most restricted ranges due to climate change (Schloss et al. 2012), forcing them to utilize a variety of possible, non-preferred habitats
Small-Scale Change
On a small or local scale, the effects of climate change are more fine-tuned and can differ depending on the species. For example, spider monkeys (Ateles geoffroyi yucatanensis) exhibited behavioral plasticity after two hurricanes hit Mexico, spending more time resting, feeding on leaves, and in smaller subgroups than they did before the hurricanes (Schaffner et al. 2012). Critically endangered species, such as the northern sportive lemur (Lepilemur septentrionalis), are more vulnerable to catastrophic weather events because in small populations the loss of just a few individuals can have major impacts (Figure B.12) (Dinsmore et al. 2016). Species that are not threatened or that have large, intact ranges are not likely to be greatly affected by localized climatic conditions, but they may nonetheless experience local devastation and even extinction (Strier 2017).

Disease
Disease is especially intertwined with climate change and has increasingly become a critical threat to primates (Nunn and Altizer 2006). Shifting temperatures, unpredictable precipitation, crowding in fragmented habitats, and increased human contact can contribute to increased disease transmission among primates (Nunn and Gillespie 2016). Mosquito populations, which are vectors of diseases that affect humans and nonhuman primates like Zika virus, yellow fever, and malaria, often thrive in this type of environment and have expanded in recent years (Lafferty 2009). Disease outbreaks have the potential to severely reduce primate populations. In 2016 and 2017, a large yellow fever outbreak devastated several populations of the brown howler monkey (Alouatta guariba) endemic to the Atlantic forest of Brazil (Fernandes et al. 2017). Ebola outbreaks have similarly diminished populations of African apes; in 2003 and 2004, an outbreak killed up to 5,000 endangered western gorillas (Gorilla gorilla) (Bermejo et al. 2006) and severely reduced populations of chimpanzees (Pan troglodytes) (Leroy et al. 2004) in Gabon and the Republic of Congo.
Human encroachment into primate habitats as a result of agricultural expansion, resource extraction, or even through irresponsible eco-tourism or research practices can introduce novel pathogens into both human and nonhuman primate populations (Strier 2017). Due to our close shared lineage, many diseases are communicable between humans and primates, such as Ebola, HIV, tuberculosis, herpes, and other common ailments. Close contact and primate handling are often the most direct ways in which these diseases are transmitted. However, poor hygiene practices, improper waste disposal, and primate provisioning contribute to disease susceptibility in primates (Wallis and Lee 1999). For example, two groups of olive baboons (Papio cynocephalus anubis) living in the Masai Mara Game Reserve in Kenya contracted tuberculosis from foraging at contaminated garbage dumps near the tourist lodge (Tarara et al. 1985). Similarly, in Gombe National Park, Tanzania, there is evidence that contact with humans and their domesticated animals in nearby villages caused an outbreak of polio in chimpanzees (Pan troglodytes) (Williams et al. 2008). Transmission of diseases through increased human contact can have devastating effects on primate populations that have not built any resistance (Laurance 2015).
Extinction Vortex

The many threats facing primates that we have listed here are completely interrelated. As such, they tend to interact with one another, creating what is known as an extinction vortex (Figure B.13; Gilpin and Soulé 1986). Habitat fragmentation and loss, hunting, climate change, and disease compound to reduce primate populations at a greater rate than when acting alone. Small populations living in isolated fragments of habitat are disconnected from the rest of their species and are therefore more vulnerable to inbreeding effects. Daniel Brito and colleagues (2008) found that many populations of the critically endangered northern muriqui (Brachyteles hypoxanthus) residing in the remaining fragments of the Atlantic Forest would experience genetic decay with possibility of extinction over the next 50 generations if management practices were not put into place. Slow life histories resulting in long interbirth intervals push many species of primates farther into the extinction vortex. Shifting demographics can have dire consequences for primates, thrusting them into a cycle that is hard to break once entered. With the continued presence of threats, many species have a difficult time recovering (Brook et al. 2008; Strier 2011a).
PRIMATE SIGNIFICANCE
Ecological Significance of Primates
Primates play a key role within their ecosystems, often acting as important contributors to forest community structure by aiding in seed dispersal and pollination of angiosperms and other plant species. Variability in traits such as diet, gut anatomy, and movement patterns influence the spatial landscape of dispersed seeds (Russo and Chapman 2011). Frugivorous primates that range widely are considered the greatest contributors to the dispersal of seeds, as they often either swallow seeds whole, as is common for most Neotropical frugivorous primates (Figure B.14), or spit seeds out, as is common for primates with cheek pouches in Africa and Asia. These primates can contribute greatly to the diversification and regeneration of forest communities by traveling long distances after consumption and depositing seeds away from the parent plant within heterogeneous landscapes (Strier 2017; Terborgh 1983). Frugivory and seed dispersal are critical plant-animal relationships (Russo 2017). Bach Thanh Hai and colleagues (2018) found that yellow-cheeked crested gibbons (Nomascus gabriellae) in Southeast Asia were the most effective seed disperser for the Pacific walnut tree. Gibbons dispersed seeds via consumption anywhere from 4 m to 425 m from the parent tree. Seeds defecated by gibbons had higher germination and success rates than those spit by macaques in the same forest.
Some species of primate may also act as pollinators for local plant species. These primates are attracted to the nectar and flowers of the plant, which often leave pollen on their faces and fur, subsequently spreading pollen to conspecifics when the primate moves to a new location. Jeremy Hogan and colleagues (2016) suggest that white-faced capuchins (Cebus capucinus imitator) may have a beneficial effect on certain plant species in Costa Rica. Other primates may have co-evolved a plant-pollinator relationship. Data indicate that the black and white ruffed lemur (Varecia variegata) is reliant on the nectar of the traveler’s palm (Ravenala madagascariensis) during specific times of the year, allowing pollen to stick to the ruff of their necks. This, along with the notion that no other species visit the travel’s palm during certain times of the year, indicate that this plant species may be dependent on nonflying mammals for pollination (Kress et al. 1994).
By acting as seed dispersers and pollinators, primates can aid in the reproductive success, regeneration, and diversification of plants within their ecosystems. The significance of these relationships is only becoming more apparent as habitats continue to be fragmented and destroyed. As habitats dwindle, the ability to regenerate healthy forest systems is crucial to the health and survival of tropical forest systems worldwide (Stier 2017).
Bioanthropological Significance of Primates

The study of non-human primates has been an integral component of anthropology for many decades. Even before Sherwood Washburn advocated in The New Physical Anthropology (1951) that primates could be studied as living reference for hominin behaviors, anthropologists like Margaret Mead recognized that studies of wild primates could contribute to biological and sociocultural anthropology in many ways (Strier 2011b). Primatology in Japan, the U.S., and Europe grew out of a desire to better understand ourselves. Thus, research in the 1960s and 1970s largely focused on species such as chimpanzees (Pan spp.) or baboons (Papio spp.) that are closely related to humans phylogenetically or live in environments similar to those occupied by early hominins (Haraway 1991; Strum and Fedigan 1999; Washburn 1973). Since those early days, biological anthropological primatology has broadened to include primates from around the world (Strier 2003, 2018a). The inclusion of diverse taxa from what were then-understudied regions challenged notions of “typical” primate behavior, such as the idea that aggression was the main mechanism for maintaining hierarchical social relationships (Strier 1994).
Anthropologists draw broadly from primate studies to explore the many facets of human behavior and evolution. For example, studies demonstrating the tool-using capabilities of wild chimpanzees (Pan troglodytes) and capuchin monkeys (Sapajus spp., formerly Cebus spp.) show that similar ecological pressures and intelligence contribute to tool-using behaviors, rather than just phylogenetic relatedness to humans (Fragaszy et al. 2004; Inoue-Nakamura and Matsuzawa 1997). Similarly, studies of modern primate morphology are frequently used to assess how locomotor style or behaviors (such as foraging) are related to anatomy, and this knowledge can then be used to assess the skeletal and dental anatomy of fossil hominins. Studies of microscopic wear patterns, for example, use living primates as analogues to understand wear patterns generated by different food items as well as the rapidity with which changes to these patterns can occur (Teaford and Oyen 1989; Ungar and Sponheimer 2011). Living nonhuman primates provide a comparative sample with which we deepen our understanding of the evolutionary mechanisms that shaped human evolution.
Cultural Significance of Primates
For as long as our species has existed, groups of people have lived alongside nonhuman primates and engaged with them in varying ways (Fuentes 2012). The development and expansion of the field of ethnoprimatology, the study of the human–nonhuman primate interface, has encouraged researchers from sociocultural anthropology and primatology to investigate these points where primates and humans interact and influence each other in surprising ways (Fuentes 2012; Sponsel 1997). Primates are viewed by many as exceptional animals for the ways in which they reflect elements of humanness, stimulating many thousands of people to observe their exhibits at zoos and sanctuaries throughout the world. However, the significance of these animals to diverse cultures goes beyond anthropocentrism and touches on aspects of ecology, religion, and social systems. Primates are common figures in religion and myth, appearing sometimes as gods or deities themselves (e.g., the Hindu deity Hanuman) and sometimes as mediators between the human and spirit realms (Alves et al. 2017; Peterson 2017; Wheatley 1999). Primates have additional cultural significance as figures in folklore and legend, and they are often ascribed human-like characteristics in many of these narratives (Cormier 2017). These stories often inform local taboos that may discourage the consumption of particular species or deforestation of particular areas (Osei-Tutu 2017; Roncal et al. 2018; Sicotte 2017).
The role that primates play in human cultures is complex and varies significantly with local history, religious practice, and economies. Among the Awa Guajá of eastern Amazonia, for example, primates are considered a part of the humans’ extended kin network and are protected as such, yet they also constitute an important source of dietary protein and are hunted regularly (Cormier 2003). In other primate habitat countries, such as Bali, primates play a significant role in religious practice. Long-tailed macaques (Macaca fascicularis) in Bali are frequently found in the forests surrounding Hindu temples and will consume offerings left by residents and tourists once festivals or rituals are concluded (Fuentes 2010; Wheatley 1999). These macaques are seen by some as mediators between the natural world and the spiritual world that transports offerings from one realm to another (Wheatley 1999). Investigating how local residents view primates—for example, whether species are considered sacred or not—is a vital component of conservation programs in these areas (Peterson and Riley 2017). Studying the interface between human and nonhuman primates, and what factors (e.g., local religious practices, taboos, etc.) influence these interactions can lead to more holistic conservation planning and implementation.
Economic Significance of Primates
One of the most promising ways that primates can benefit people is through the potential to stimulate local economies from ecotourism. Ecotourism differs from traditional tourism in three main ways: it focuses on nature-based attractions, it provides learning opportunities, and its tourism management practices adhere to economic and ecological sustainability (Fennell and Weaver 2005). Primates are charismatic megafauna, meaning that they are large animals (oftentimes mammals) that elicit mass appeal. They have the possibility to draw tourists, which can in turn bring revenue to lower-income communities found near primate habitats. This attraction from tourists, along with revenue-sharing, can then stimulate local populations to have more positive attitudes toward protected areas and become more invested in the well-being and protection of primates and their habitats (Archabald and Naughton-Treves 2001).

Perhaps one of the greatest success stories of nature-based tourism revolves around the mountain gorillas (Gorilla beringei beringei) of Rwanda. After internal conflict plagued Rwanda during the 1990s, the Virungas area developed gorilla-based tourism as a means to aid in socioeconomic development and to bring stability to the region. This process not only helped to increase mountain gorilla populations but was also able to generate enough income to cover the operation costs of three national parks (Maekawa et al. 2013). Research indicated that low-income individuals living around Parc National des Volcans in Rwanda could garner direct income as well as nonfinancial benefits (such as the development of schools and hospitals) from gorilla tourism in the region (Spencely et al. 2010). Although ecotourism has the potential to alleviate poverty situations for local populations and aid in the overall sustainability of natural habitats, it can also bring a suite of new problems to areas. It can overcrowd national parks and over-habituate primates (Figure B.15), increase potential disease transfer between humans and primates, and exacerbate corruption, which often pulls money away from local communities (Hvenegaard 2014; Muehlenbein et al. 2010).
WHAT CAN BE DONE?
Role of Research
Systematic and long-term research studies provide some of the most foundational and necessary information for the conservation of endangered primates (Kappeler and Watts 2012). Research provides critical data on essential and preferred feeding resources, life history parameters and reproduction rates, territoriality, the carrying capacity of habitats, and solitary or group social dynamics. Within the last few decades, researchers have also begun to stress the acute need for studies investigating how various primates are responding to human disturbances; how climate change is affecting the behavior, range, and habitat of these species; and the significance of primate biodiversity hotspots (Brown and Yoder 2015; Chapman and Peres 2001; Estrada et al. 2018). Understanding these aspects will provide crucial information for practitioners to make the most effective and species-specific conservation decisions.
Long-term studies on primate species provide some of the most conclusive information on changes occurring to populations in the face of anthropogenic disturbances and climate change. They also provide a suite of direct and indirect conservation contributions to endangered species, and the continual monitoring of populations can deter deleterious anthropogenic actions, allowing for population growth and forest regeneration. For example, the Northern Muriqui Project of Caratinga in Minas Gerais, Brazil, has documented growth of both the muriqui population and the regeneration of the forest via secondary succession (Strier 2010). The project has also invested in future research and conservation by training more than 65 Brazilian students, as well as providing stable jobs for local people, stimulating the local community, and alleviating reliance on forest products for income and survival (Strier 2010; Strier and Boubli 2006; Strier and Mendes 2012). Several other long-term primate studies all over the world have seen similar positive impacts and conservation successes including work in Beza Mahafaly Special Reserve, Berenty Reserve, and Ranomafana National Park, Madagascar; Lomas Barbudal and Santa Rosa National Park, Costa Rica; Khau Yai National Park, Thailand; Amboseli National Park, Kenya; Gombe Stream National Park, Tanzania; Kibale National Park, Uganda, among many others (Kappeler and Watts 2012).
The implementation of novel research techniques can also aid in the conservation of primates and their ecosystems. The use of high-resolution camera traps have become widespread and invaluable in their ability to aid primatologists and conservationists in surveying rare populations, establishing population counts, and assessing behavior (Pebsworth and LaFleur 2014). Camera traps also have the potential to aid in ethical considerations, photographing illegal activities such as poaching. Research is also imperative for making important decisions regarding translocations and reintroductions of animals. Without knowledge of the species’ social ecology, demography, and unique learned behaviors—also known as primate traditions or cultures—successful translocations and reintroductions from captive populations would not be possible. Researchers and conservationists must recognize these dynamics when making the difficult decision to reintroduce or move populations and factor in how these dynamics may shift or affect the resident population after management. The most notable case of effective translocation and reintroduction is that of the golden lion tamarin (Leontopithecus rosalia). Over 30 zoos contributed 146 captive-born individuals to be reintroduced into Brazil, providing essential information on nutrition and health that aided in reintroduction strategies. Additionally, in 1994, isolated individuals in forest fragments were successfully translocated into protected regions in order to increase gene flow, which through the exchange of genes, introduces more genetic variation into the next generation (Kierulff et al. 2012).
Nongovernmental Organizations (NGOs) and Community-Based Conservation Work
Conservation NGOs have a long-standing history of working to save endangered species from going extinct. These organizations often target primates for their work because of their ability to act as umbrella species, supporting the conservation of many species found within their ecosystems. Over the past 30 years, conservation NGOs have begun to move away from a preservation-based mindset that focused on excluding humans from using protected areas. The 1990s ushered in a shift toward community-based conservation (CBC), which instead aimed to work with local people living near targeted natural environments to reduce human-wildlife conflict and establish sustainable practices (Horwich and Lyon 2007). CBC has shown success in terms of reducing hunting and deforestation in many regions including the Manas Biosphere Reserve in Assam, India, as well as in the cloud forests of Peru from the work of the Yellow Tailed Woolly Monkey Project (Horwich et al. 2012; Shanee et al. 2007). Although CBC has seen conservation successes, many warn that it should not be a panacea for all conservation goals but, rather, one mechanism among many when attempting to conserve endangered species (Reibelt and Nowack 2015; Scales 2014).
Reforestation is widely becoming one of the most practical ways in which NGOs aid in primate conservation. Organizations often collaborate with communities to establish nurseries to grow saplings, which can then be transplanted strategically to reforest certain parts of primate habitats or create habitat corridors between forest fragments. Madagascar Biodiversity Partnership, an NGO with four field sites throughout Madagascar, has planted over 1,850,000 trees from 2010 to June of 2018 (Edward E. Louis Jr., personal communication, 05/24/2018). These efforts have been shown to be successful, as lemurs have been observed in reforested regions where they had previously not been seen.
What Can Readers of This Book Do?
It may be difficult to imagine how an individual living thousands of miles away can aid in the conservation of primates and their habitats, but in fact there are several small steps that people all over the world can take to make a difference. Many local zoos contribute to in situ conservation work as well as maintain species survival plans in order to increase diversity among zoo populations. We recommend readers visit their local zoos to learn about what actions zoos take to aid in the conservation of primates and how they can get involved in these activities.
One tangible action that can be done is to reduce the purchasing of products that contain non-sustainable ingredients. The demand for cheap oil has increased in recent years for commercial products such as peanut butter, chocolate, soaps, and shampoos, among many others. As such, palm oil plantations have expanded into wildlife habitat throughout Southeast Asia, especially in Borneo and Sumatra, the last remaining habitats of orangutans (Pongo spp.) and many other species of primates. This, coupled with other local pressures such as hunting and peat fires, resulted in the IUCN upgrading the Borneo orangutan’s (Pongo pygmaeus) conservation status to Critically Endangered in 2016. They were also included in the 25 most endangered primates list for the first time (Husson et al. 2016). Although data suggest that orangutans will nest within agroindustrial environments, they will only do so with natural forest patches nearby (Ancrenaz et al. 2014). Reducing individual consumption of palm oil or choosing sustainable oil products can help reduce the overall demand; it can drive producers to commit to more environmentally friendly practices. This can hopefully slow the conversion of naturally forested landscapes into agroindustrial environments.
With the proliferation of social media, the desirability to photograph animals in close proximity has greatly increased (Pearce and Moscardo 2015). We recommend that readers who visit native primate environments resist engaging with primates in an attempt to take “selfies” with animals. Repeated encounters with travelers and tourists can overhabituate primates and put them in danger of contracting (and transmitting) diseases (Geffroy et al. 2015). Paying for photos with primates can also exacerbate the illegal pet trade because local people will be incentivized to harvest primate infants from wild populations, adversely affecting primate densities and social group dynamics. While it may be popular to try to take the most engaging “selfie” with a wild animal, it is best to just admire these animals from afar (Figure B.16).

Lastly, readers can aid in primate conservation by resisting sharing YouTube (or other) videos depicting primates in nonnative habitats. Often times videos of primates engaging with humans spark the popularity of these animals as pets. The desire for these animals can lead to an influx in illegal pet harvesting and trading, the mistreatment of wild animals in domestic settings, and the belief that these animals are not endangered since others own them as pets (Nekaris et al. 2013). After a video depicting a pygmy slow loris (Nycticebus pygmaeus) being “tickled” went viral in 2009, and another depicting a slow loris eating rice went viral in 2012, international confiscations of slow lorises increased (Nekaris et al. 2013). Awareness, coupled with the resistance to share these “cute” videos, can help reduce the market for primates to be captured for the illegal pet trade.
For those interested in gaining hands-on experience with primates, we recommend visiting Primate Info Net, where a list of field school opportunities and professional, educational, and volunteer positions are posted regularly. These listings can be found at: http://pin.primate.wisc.edu/jobs/list/avail
FUTURE PERSPECTIVES
As anthropogenic and natural disturbances continue to intensify in range and scale, the future status of the world’s primates is increasingly dire. However, researchers, conservationists, and the general public are attempting to understand how primates respond to these disturbances, what actions can be done to mitigate further disturbances, how to establish sustainable relationships between humans and primates, and what small actions can be done in their own lives to aid these processes.
Regardless of our cultural or political views, we think it is valid to ask ourselves as researchers, conservationists, and students: What is the value of Earth’s biological diversity, and what are our obligations to nonhuman primates, our closest living ancestors? Although scientists and conservationists often argue that there is inherent value in maintaining the world’s biodiversity, we propose that primates have a special significance that goes beyond their intrinsic contribution to biodiversity. The concept that species and systems can provide a suite of benefits to humans is known as ecosystem services (Cardinale et al. 2012; Kremen 2005). These services are often classified into four categories: provisioning (e.g., food), regulating (e.g., water quality regulating), cultural (e.g., recreation and aesthetic), and supporting services (e.g., nutrient cycling) (Harrison et al. 2014; Mace et al. 2011; Millennium Ecosystem Assessment 2005). Following this approach, we propose that understanding the value of primates and their habitats in terms of their ecological, bioanthropological, cultural/historical, and economic contributions can aid in the long-term conservation of these endangered species. Recognizing the connections and continuities between ourselves and other primates is the first critical step toward caring about their future and making it part of our own.
Review Questions
- What criteria do researchers and conservationists use to identify the conservation status of primate populations and species?
- What are the main threats facing primates today, and how do the combined impacts of these threats uniquely affect primates?
- What do you think a world without primates would look like? Consider their unique significance and the various roles they play in ecology, human evolutionary and cultural history, and local economies. How would the absence of primates affect ecosystems, other animals, and humans?
- Considering all the other problems in the world today, should primate conservation be a high priority? What are the arguments to support prioritizing primate conservation?
- How can you contribute to primate conservation in your everyday life?
About the Authors
Mary P. Dinsmore, M.S., Ph.D. candidate
University of Wisconsin–Madison

Mary P. Dinsmore is a Ph.D. candidate in the Department of Environment and Resources at the University of Wisconsin–Madison and a member of the Strier Lab. Mary’s interest in primatology began when she was working as a research assistant in Peru with saddleback tamarins (Saguinus fuscicollis) and in Madagascar with greater bamboo lemurs (Prolemur simus). It was during these experiences that she saw firsthand the immense impacts that humans had on primate habitat and became interested in human-wildlife conflict and conservation. Her dissertation work explores the consequences of anthropogenic and natural disturbances on the habitat and behavior of the northern sportive lemur (Lepilemur septentrionalis). She has received funding for this work from the Primate Action Fund of Conservation International and African Studies Department of UW–Madison. Mary received her BS and BA from the University of Portland in 2009 and her MS from the University of Wisconsin–Madison in 2014. She currently teaches study abroad with Broadreach Global Adventures as well as throughout the academic year in the Department of Integrative Biology at UW–Madison.
Ilianna E. Anise, B.A., Ph.D. student
University of Wisconsin–Madison

Ilianna E. Anise is a Ph.D. student in the Integrative Biology department at the University of Wisconsin–Madison. She is a member of the Strier Lab, studying primate behavioral ecology and conservation. Ilianna’s current research focuses on studying vigilance and risk in black-horned capuchin monkeys (Sapajus nigritus) in southeast Brazil. She holds an Advanced Opportunity Fellowship and has received funding from the Department of Integrative Biology. Ilianna received her BA in biology and environmental science from Drew University. She found her passion for fieldwork while participating in a small mammal demography research project as an undergraduate student. At UW–Madison, Ilianna is a teaching assistant for an animal biology lab course and enjoys sharing her love of animals with students.
Rebekah J. Ellis, B.A., M.S.
University of Wisconsin–Madison

Rebekah J. Ellis is a Ph.D. student in the Department of Anthropology at the University of Wisconsin–Madison. She is a member of the Strier Lab. Rebekah received her BA in anthropology and psychology from the University of Texas at Austin. She has studied the behavior of neotropical primates at field sites in Eastern Costa Rica and the Ecuadorian Amazon. At UW–Madison, Rebekah has assisted in teaching an introductory course covering the subdisciplines of cultural, archeological, and biological anthropology. She currently holds a Foreign Language and Area Studies Fellowship. Her research utilizes social network analysis to explore the social behavior of neotropical primates.
Amanda J. Hardie, M.A., Ph.D. candidate
University of Wisconsin–Madison

Amanda J. Hardie is a Ph.D. Candidate in Anthropology at the University of Wisconsin–Madison and a member of the Strier Lab. Amanda received her BA in Anthropology at the University of North Carolina-Charlotte, after attending Central Piedmont Community College. Amanda’s research focuses on the dynamic interactions between primates and the environment, and she currently works in Brazil studying the behavior and diet of brown howler monkeys in the Atlantic Forest. She has received funding from the Department of Anthropology, a Foreign Language Area Studies Fellowship, and the National Geographic Society.
Jacob B. Kraus, B.A., Ph.D. student
University of Wisconsin–Madison

Jacob B. Kraus is a Ph.D. student and teaching assistant in the Department of Integrated Biology at the University of Wisconsin–Madison and a member of the Strier Lab. His interest in primatology began while studying the ecology and behavior of Gelada monkeys (Theropithecus gelada) as a field assistant for the Guassa Gelada Research Project (GGRP) in Ethiopia. He is broadly interested in the behavioral thermoregulation strategies that primates, and other social mammals, employ in high-altitude habitats. Jacob’s current research is focused on how the sociality of Yunnan snub-nosed monkeys (Rhinopithecus bieti) affects their microhabitat selection and grooming behaviors. He has been funded by the Department of Integrative Biology. Jacob received his BA in Biology from Reed College. Prior to attending UW–Madison, Jacob interned at the Smithsonian Conservation Biology Institute (SCBI), where he worked on various remote sensing and habitat survey projects.
Karen B. Strier, Ph.D.
University of Wisconsin–Madison

Karen B. Strier is Vilas Research Professor and Irven DeVore Professor of Anthropology at the University of Wisconsin–Madison. After graduating from Swarthmore College in 1980, she received her MA in 1981 and her PhD in 1986 in Anthropology from Harvard University. She is an international authority on the endangered northern muriqui monkey, which she has been studying in the Brazilian Atlantic forest since 1982. Her pioneering, long-term field research has been critical to conservation efforts on behalf of this species, and has been influential in broadening comparative perspectives on primate behavioral and ecological diversity. She is a fellow of the U.S. National Academy of Sciences, the American Academy of Arts and Sciences, and the American Association for the Advancement of Science. She was awarded an Honorary Doctorate of Science from the University of Chicago, and Distinguished Primatologist Awards from the American Primatological Society and the Midwestern Primate Interest Group. She has received various research, teaching, and service awards from the University of Wisconsin–Madison and has been honored with Lifetime Honorary Memberships from the Brazilian Primatological Society and the Latin American Primatological Society, and with Honorary Citizenship of the city of Caratinga, in Minas Gerais, Brazil. She has authored or coauthored more than 100 publications, in addition to working on various coauthored and edited volumes and two single-authored books, Faces in the Forest: The Endangered Muriqui Monkeys of Brazil (1992) and Primate Behavioral Ecology, 5th edition (2017). Since 2016, she has served as the elected President of the International Primatological Society.
References
Aide, T. Mitchell, Matthew L. Clark, H. Ricardo Grau, David López‐Carr, Marc A. Levy, Daniel Redo, Martha Bonilla‐Moheno, George Riner, María J. Andrade‐Núñez, and María Muñiz. 2013. “Deforestation and Reforestation of Latin America and the Caribbean (2001–2010).” Biotropica 45 (2): 262–271. doi: 10.1111/j.1744-7429.2012.00908.x.
Alves, Rômulo Romeu Nóbrega, Raynner Rilke Duarte Barboza, and Wedson de Medeiros Silva Souto. 2017. “Primates in Mythology.” In The International Encyclopedia of Primatology, edited by Agustín Fuentes, 1149–1154. Hoboken, NJ: John Wiley and Sons. doi: 10.1002/9781119179313.wbprim0173.
Ancrenaz, M., M. Gumal, A. J. Marshall, E. Meijaard, S. A. Wich, and S. Husson. 2016. Pongo pygmaeus (errata version published in 2018). The IUCN Red List of Threatened Species 2016. doi: 10.2305/IUCN.UK.2016-1.RLTS.T17975A17966347.
Ancrenaz, Marc, Felicity Oram, Laurentius Ambu, Isabelle Lackman, Eddie Ahmad, Hamisah Elahan, Harjinder Kler, Nicola K. Abram, and Erik Meijaard. 2014. “Of Pongo, Palms, and Perceptions: A Multidisciplinary Assessment of Bornean Orangutans Pongo pygmaeus in an Oil Palm Context.” Oryx 49 (3): 465–472. doi: 10.1017/S0030605313001270.
Anderson, Jill T., David W. Inouye, Amy M. McKinney, Robert I. Coulatti, and Tom Mitchell-Oldes. 2012. “Phenotypic Plasticity and Adaptive Evolution Contribute to Advancing Flowering Phenology in Response to Climate Change.” Proceedings of the Royal Society B 279 (1743): 3843–3852. doi: 10.1098/rspb.2012.1051.
Archabald, Karen, and Lisa Naughton-Treves. 2001. “Tourism Revenue-Sharing around National Parks in Uganda: Early Efforts to Identify and Reward Local Communities.” Environmental Conservation 28 (2): 135–149. doi: 10.1017/S0376892901000145.
Behie, Alison M., and Mary S. M. Pavelka. 2005. “The Short-Term Effects of a Hurricane on the Diet and Activity of Black Howlers (Alouatta pigra) in Monkey River, Belize.” Folia Primatologica 76 (1): 1–9. doi: 10.1159/000082450.
Bermejo, Magdalena, Jose Domingo Rodriguez-Teijeiro, German Illera, and Peter D. Walsh. 2006. “Ebola Outbreak Killed 5,000 Gorillas.” Science 314 (5805): 1564. doi: 10.1126/science.1133105.
Brito, Daniel, Carlos E. V. Grelle, and Jean Phillipe Boubli. 2008. “Is the Atlantic Forest Protected Area Network Efficient in Maintaining Viable Populations of Brachyteles hypoxanthus?” Biodiversity and Conservation 17 (13): 3255–3268. doi: 10.1007/s10531-008-9427-z.
Brook, Barry W., Navjot S. Sodhi, and Corey J. A. Bradshaw. 2008. “Synergies among Extinction Drivers under Global Change.” Trends in Ecology and Evolution 23 (8): 453–460. doi: 10.1016/j.tree.2008.03.011.
Brown, Jason L., and Anne D. Yoder. 2015. “Shifting Ranges and Conservation Challenges for Lemurs in the Face of Climate Change.” Ecology and Evolution 5 (6): 1131–1142. doi: 10.1002/ece3.1418.
Campos, Fernando A., Katharine M. Jack, and Linda M. Fedigan. 2015. “Climate Oscillations and Conservation Measures Regulate White-Faced Capuchin Population Growth and Demography in a Regenerating Tropical Dry Forest in Costa Rica.” Biological Conservation 186: 204–213. doi: 10.1016/j.biocon.2015.03.017.
Campos, Fernando A., William F. Morris, Susan C. Alberts, Jeanne Altmann, Diane K. Brockman, Marina Cords, Anne Pusey, Tara S. Stoinski, Karen B. Strier, and Linda M. Fedigan. 2017. “Does Climate Variability Influence the Demography of Wild Primates? Evidence from Long-Term Life-History Data in Seven Species.” Globe Change Biology 23 (11): 1–15. doi: 10.1111/gcb.13754.
Cardinale, B. J., J. Emmett Duffy, Andrew Gonzalez, David U. Hopper, Charles Perrings, Patrick Venail, Anita Narwani, et al. 2012. “Biodiversity Loss and Its Impact on Humans.” Nature 486 (7401): 59–67. doi: 10.1038/nature11148.
Carlson, Kimberly M., Lisa M. Curran, Dessy Ratnasari, Alice M. Pittman, Britaldo S. Soares-Filho, Gregory P. Asner, Simon N. Trigg, David A. Gaveau, Deborah Lawrence, and Herman O. Rodrigues. 2012. “Committed Carbon Emissions, Deforestation, and Community Land Conversion from Oil Palm Plantation Expansion in West Kalimantan, Indonesia.” Proceedings of the National Academy of Sciences 109 (19): 7559–7564. doi: 10.1073/pnas.1200452109.
Chapman, Colin A., and Carlos A. Peres. 2001. “Primate Conservation in the New Millennium: The Role of Scientists.” Evolutionary Anthropology 10 (1): 16–33. doi: 10.1002/1520-6505(2001)10:1<16::AID-EVAN1010>3.0.CO;2-O.
CITES. N.d. “CITES Trade Database.” Accessed July 22, 2018. https://trade.cites.org/en/cites_trade/#
Clark, Natalie E., Elizabeth H. Boakes, Philip J. K. McGowan, Georgina M. Mace, and Richard A. Fuller. 2013. “Protected Areas in South Asia Have Not Prevented Habitat Loss: A Study Using Historical Models of Land-Use Change.” PLoS ONE 8 (5):e65298. doi: 10.1371/journal.pone.0065298.
Clauzel, Celine, Deng Xiqing, Wu Gongsheng, Patrick Giraudoux, and Li Li. 2015. “Assessing the Impact of Road Developments on Connectivity across Multiple Scales: Application to Yunnan Snub-Nosed Monkey Conservation.” Biological Conservation 192: 207–217. doi: 10.1016/j.biocon.2015.09.029.
Conaway, Eileen. 2011. “Bioidentical Hormones: An Evidence-Based Review for Primary Care Providers.” The Journal of the American Osteopathic Association 111 (3): 153–164.
Cormier, Loretta A. 2003. Kinship with Monkeys: The Guajá Foragers of Eastern Amazonia. New York: Columbia University Press.
———. 2017. “Primates in Folklore.” In The International Encyclopedia of Primatology, edited by Agustín Fuentes, 1139–1146. Hoboken, NJ: John Wiley and Sons. doi: 10.1002/9781119179313.wbprim0285.
Covey, Ryan, and W. Scott McGraw. 2014. “Monkeys in a West African Bushmeat Market: Implications for Cercopithecid Conservation in Eastern Liberia.” Tropical Conservation Science 7 (1): 115–125. doi: 10.1177/194008291400700103.
Cronin, Drew T., Stephen Woloszynek, Wayne A. Morra, Shaya Honarvar, Joshua M. Linder, Mary Katherine Gonder, Michael P. O’Connor, and Gail W. Hearn. 2015. “Long-term Urban Market Dynamics Reveal Increased Bushmeat Carcass Volume Despite Economic Growth and Proactive Environmental Legislation on Bioko Island, Equatorial Guinea.” PLoS ONE 10 (7): e0134464. doi: 10.1371/journal.pone.0134464.
Dinsmore, Mary P., Edward E. Louis Jr., Daniel Georges Randriamahazomana, Ali Hachim, John R. Zaonarivelo, and Karen B. Strier. 2016. “Variation in Habitat and Behavior of the Northern Sportive Lemur (Lepilemur septentrionalis) at Montagne des Français, Madagascar.” Primate Conservation 30: 73–88.
Dinsmore, Mary P., Karen B. Strier, and Edward E. Louis Jr. 2018. “The Impacts of Cyclone Enawo and Anthropogenic Disturbances on the Habitat of Northern Sportive Lemurs (Lepilemur septentrionalis) in Northern Madagascar.” American Journal of Physical Anthropology 165 (2): 68.
Dunham, Amy E., Elizabeth M. Erhart, Deborah J. Overdorff, and Patricia C. Wright. 2008. “Evaluating Effects of Deforestation, Hunting, and El Nino Events on a Threatened Lemur.” Biological Conservation 141 (1): 287–297. doi: 10.1016/j.biocon.2007.10.006.
Eppley, Timothy M., Giuseppe Donati, Jean Baptiste Ramanamanjato, Faly Randriatafika, Laza N. Andriamandimbiarisoa, David Rabehevitra, Robertin Ravelomanantsoa, and Jörg U. Ganzhorn. 2015. “The Use of an Invasive Species Habitat by a Small Folivorous Primate: Implications for Lemur Conservation in Madagascar.” PLoS ONE 10 (11): e0140981. doi: 10.1371/journal.pone.0140981.
Estrada, Alejandro, Paul A. Garber, Russell A. Mittermeier, Serge Wich, Sidney Gouveia, Ricardo Dobrovolski, K. A. I. Nekaris, et al. 2018. “Primates in Peril: The Significance of Brazil, Madagascar, Indonesia, and the Democratic Republic of the Congo for Global Primate Conservation.” PeerJ 6: e4869. doi: 10.7717/peerj.4869.
Estrada, Alejandro, Paul A. Garber, Anthony B. Rylands, Christian Roos, Eduardo Fernandez-Duque, Anthony Di Fiore, K. Anne-Isola Nekaris, et al. 2017. “Impending Extinction Crisis of the World’s Primates: Why Primates Matter.” Science Advances 3(229): 1–16. doi: 10.1126/sciadv.1600946.
Fa, John E., Jesus Olivero, Miguel A. Farfán, Ana L. Márquez, Jesús Duarte, Janet Nackoney, Amy Hall, et al. 2015. “Correlates of Bushmeat in Markets and Depletion of Wildlife.” Conservation Biology 29 (3): 805–815. doi: 10.1111/cobi.12441.
Farias, Izeni P., Wancley G. Santos, Marcelo Gordo, and Tomas Hrbek. 2015. “Effects of Forest Fragmentation on Genetic Diversity of the Critically Endangered Primate, the Pied Tamarin (Saguinus Bicolor): Implications for Conservation.” Journal of Heredity 106 (S1): 512–521. doi: 10.1093/jhered/esv048.
Fennell, David, and David Weaver. 2005. “The Ecotourium Concept and Tourism-Conservation Symbiosis.” Journal of Sustainable Tourism 13(4): 373–390. doi: 10.1080/09669580508668563.
Fernandes, Natalia C.C.A., Mariana Sequetin Cunha, Juliana Mariotti Guerra, Rodrigo Albergaria Ressio, Cinthya dos Santos Cirqueira, Silvia D’Andretta Iglezias, Julia de Carvalho, Emerson L.L. Aruajo, Jose-Luiz Catao-Dias, and Josue Diaz-Delgado. 2017. “Outbreak of Yellow Fever among Nonhuman Primates, Espirito Santo, Brazil, 2017.” Emerging Infectious Diseases 23 (12): 2038–2041. doi: 10.3201/eid2312.170685.
Fragaszy, Dorothy, Patrícia Izar, Elisabetta Visalberghi, Eduardo B. Ottoni, and Marino Gomes de Oliveira. 2004. “Wild Capuchin Monkeys (Cebus libidinosus) Use Anvils and Stone-Pounding Tools.” American Journal of Primatology 64 (4): 359–366. doi: 10.1002/ajp.20085.
Fuentes, Agustín. 2010. “Natural Cultural Encounters in Bali: Monkeys, Temples, Tourists, and Ethnoprimatology.” Cultural Anthropology 25 (4): 600–624. doi:10.1111/j.1548-1360.2010.0170.x
———. 2012. “Ethnoprimatology and the Anthropology of the Human-Primate Interface.” Annual Review of Anthropology 41: 101–117. doi: 10.1148/annurev-anthro-092611-145808.
Fuller, Grace, Wilhelmina Frederica Eggen, Wirdateti Wirdateti, and K. A. I. Nekaris. 2018. “Welfare Impacts of the Illegal Wildlife Trade in a Cohort of Confiscated Greater Slow Lorises, Nycticebus Coucang.” Journal of Applied Animal Welfare Science 21 (3): 224–238. doi: 10.1080/10888705.2017.1393338.
Geffroy, Benjamin, Diogo S.M. Samia, Eduardo Bessa, and Daniel T. Blumstein. 2015. “How Nature-Based Tourism Might Increase Prey Vulnerability to Predators.” Trends Ecology and Evolution (Amst) 30 (12): 755–765.
Gilpin, Michael E., and Michael E. Soulé. 1986. “Minimum Viable Populations: Processes of Species Extinction.” In Conservation Biology: The Science of Scarcity and Diversity, edited by Michael E. Soulé, 19–34. Sunderland, UK: Sinauer and Associates.
Groves, Colin P. 2014. “Primate Taxonomy: Inflation or Real?” Annual Review of Anthropology 43: 27–36. doi: 10.1146/annurev-anthro-102313-030232.
Hai, Bach Thanh, Jin Chen, Kim R. McConkey, and Salindra K. Dayananda. 2018. “Gibbons (Nomascus gabriellae) Provide Key Seed Dispersal for the Pacific Walnut (Dracontomelon dao), in Asia’s Lowland Tropical Forest.” Acta Oecologica 88: 71–79. doi: 10.1016/j.actao.2018.03.011.
Haraway, Donna. 1991. Simians, Cyborgs, and Women: The Reinvention of Nature. New York: Routledge.
Harrison, P. A., P. M. Barry, G. Simpson, J. R. Haslett, M. Blicharska, M. Bucur, R. Dunford, et al. 2014. “Linkages between Biodiversity Attributes and Ecosystem Services: A Systematic Review.” Ecosystem Services 9: 191–203. doi: 10.1016/j.ecoser.2014.05.006.
Hickey, J. R., A. Basabose, K. V. Gilardi, D. Greer, S. Nampindo, M. M. Robbins, and T .S. Stoinski. 2018. Gorilla beringei ssp. beringei. The IUCN Red List of Threatened Species 2018. doi: 10.2305/IUCN.UK.2018-2.RLTS.T39999A17989719.
Hogan, Jeremy D., Amanda D. Melin, Krisztina N. Mosdossy, and Linda M. Fedigan. 2016. “Seasonal Importance of Flowers to Costa Rican Capuchins (Cebus capucinus imitator): Implications for Plant and Primate.” American Journal of Anthropology 161 (4): 591–602. doi: 10.1002/ajpa.23059.
Horwich, Robert H., and Jonathan Lyon. 2007. “Community Conservation: Practitioners’ Answer to Critics.” Oryx 41(3): 376–385. doi: 10.1017/S0030605307001010.
Horwich, R. H., J. Lyon, and A. Bose. 2012. “Preserving Biodiversity and Ecosystems: Catalyzing Conservation Contagion.” In Deforestation around the World, edited by P. Moutinho, 283–318. Rijeka, Croatia: InTech.
Hosonuma, Noriko, Martin Herold, Veronique de Sy, Ruth S. de Fries, Maria Brockhaus, Louis Verchot, Arild Angelsen, and Erika Romijn. 2012. “An Assessment of Deforestation and Forest Degradation Drivers in Developing Countries.” Environmental Research Letters 7 (4). doi: 10.1088/1748-9326/7/4/044009.
Husson, Simon J., Marc Ancrenaz, Elizabeth J. Macfie, Sri Suci Utami-Atmoko, and Serge A. Wich. 2016. “Bornean Orangutan.” In Primates in Peril: The 25 Most Endangered Primates 2016–2018, edited by C. Schwitzer, R. A. Mittermeier, A. B. Rylands, F. Chiozza, E. A. Williamson, E. J. Macfie, J. Wallis, and A. Cotton, 75–78. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, Virginia.
Hvenegaard, Glen. 2014. “Economic Aspects of Primate Tourism Associated with Primate Conservation.” In Primate Tourism: A Tool for Conservation? edited by Anne E. Russon and Janette Wallis, 259–277. Cambridge, UK: Cambridge University Press.
Inoue-Nakamura, Noriko, and Tetsuro Matsuzawa. 1997. “Development of Stone Tool Use by Wild Chimpanzees (Pan troglodytes).” Journal of Comparative Psychology 111 (2): 159–173.
IPCC. 2014: “Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change”, edited by Core Writing Team, R. K. Pachauri and L. A. Meyer,151. Geneva, Switzerland: IPCC.
IUCN. 2012. “IUCN Red List Categories and Criteria: Version 3.1.” Technical Report, 32. Gland, Switzerland and Cambridge, UK: IUCN.
IUCN. 2017. “The IUCN Red List of Threatened Species: Version 13.” Available: http://www.iucnredlist.org, accessed March 3, 2017.
Kalpers, José, Elizabeth A. Williamson, Martha M. Robbins, Alastair Mcneilage, Augustin Nzamurambaho, Ndakasi Lola, and Ghad Mugiri. 2003. “Gorillas in the Crossfire: Population Dynamics of the Virunga Mountain Gorillas over the Past Three Decades.” Oryx 37 (3): 326–337. doi: 10.1017/S0030605303000589.
Kappeler, Peter M., and David P. Watts. 2012. Long-term Field Studies of Primates. New York: Springer.
Kierulff, M. C. M., C. R. Ruiz-Miranda, P. Procopio de Oliveira, B. B. Beck, A. Martins, J. M. Dietz, D. M. Rambaldi, and A. J. Baker. 2012. “The Golden Lion Tamarin Leontopithecus rosalia: A Conservation Success Story.” International Zoo Yearbook 46 (1): 36–45. doi: 10.1111/j.1748-1090.2012.00170.x.
Kremen, Claire. 2005. “Managing Ecosystem Services: What Do We Need to Know about Their Ecology?” Ecology Letters 8 (5): 468–479. doi: 10.1111/j.1461-0248.2005.00751.x.
Kress, John, George E. Schatz, Michael Andrianifahanana, and Hilary Simons Morland. 1994. “Pollination of Ravenala madagascariensis (Strelitziaceae) by Lemurs in Madagascar: Evidence for an Archaic Coevolution System?” American Journal of Botany 81 (5): 542–551. doi: 10.1002/j.1537-2197.1994.tb15483.x.
Krief, Sabrina, Philippe Berny, Francis Gumisiriza, Régine Gross, Barbara Demeneix, Jean Baptiste Fini, Colin A. Chapman, Lauren J. Chapman, Andrew Seguya, and John Wasswa. 2017. “Agricultural Expansion as Risk to Endangered Wildlife: Pesticide Exposure in Wild Chimpanzees and Baboons Displaying Facial Dysplasia.” Science of the Total Environment 598: 647–656. doi: 10.1016/j.scitotenv.2017.04.113.
Lafferty, Kevin D. 2009. “The Ecology of Climate Change and Infectious Diseases.” Ecology 90 (4): 888–900. doi: https://doi.org/10.1890/08-0079.1.
Laurance, William F., Gopalasamy Reuben Clements, Sean Sloan, Christine S. O’Connell, Nathan D. Mueller, Miriam Goosem, Oscar Venter, et al. 2014. “A Global Strategy for Road Building.” Nature 513 (7517): 229–232. doi: 10.1038/nature13717.
Laurance, William F. 2015. “Emerging Threats to Tropical Forests.” Annals of the Missouri Botanical Garden 100 (3): 159–169. doi: 10.3417/2011087.
Leroy, Eric M., Pierre Rouquet, Pierre Formenty, Sandrine Souquiere, Annelisa Kilbourne, Jean-Marc Froment, Magdalena Bermejo, et al. 2004. “Multiple Ebola Virus Transmission Events and Rapid Decline of Central African Wildlife.” Science 303 (5656): 387–390. doi: 10.1126/science.1092528.
Lewis, Rebecca, and F. Rakontondranaivo. 2011. “The Impact of Cyclone Fanele on Sifaka Body Condition and Reproduction in the Tropical Dry Forest of Western Madagascar.” Journal of Tropical Ecology 27 (4): 429–432. doi: 10.1017/S0266467411000083.
Lynch Alfaro, Jessica W., José de Sousa E. Silva Jr., and Anthony B. Rylands. 2012. “How Different Are Robust and Gracile Capuchin Monkeys? An Argument for the Use of Sapajus and Cebus.” American Journal of Primatology 74 (4): 273–286. doi: 10.1002/ajp.22007.
Mace, Georgina M., Ken Norris, and Alastair H. Fitter. 2011. “Biodiversity and Ecosystem Services: A Multilayered Relationship.” Trends in Ecology and Evolution 27 (1): 19–26. doi: 10.1016/j.tree.2011.08.006.
Maekawa, Miko, Annette Lanjouw, Eugene Rutagarama, and Douglas Sharp. 2013. “Mountain Gorilla Tourism Generating Wealth and Peace in Post-Conflict Rwanda.” Natural Resources Forum 37 (2): 127–137. doi: 10.1111/1477-8947.12020.
Maldonado, Angela M., Vincent Nijman, and Simon K. Bearder. 2009. “Trade in Night Monkeys Aotus Spp. in the Brazil-Colombia-Peru Tri-Border Area: International Wildlife Trade Regulations Are Ineffectively Enforced.” Endangered Species Research 9 (2): 143–149. doi: 10.3354/esr00209.
Millenium Ecosystem Assessment. 2005. Ecosystems and Human Well-Being: Synthesis. Washington, DC: World Resources Institute.
Muehlenbein, Michael P. 2017. “Primates on Display: Potential Disease Consequences beyond Bushmeat.” American Journal of Physical Anthropology 162 (S63): 32–43. doi: 10.1002/ajpa.23145.
Muehlenbein, Michael P., Leigh A. Martinez, Andrea A. Lemke, Laurentius Ambu, Senthilvel Nathan, Sylvia Alsisto, Rosman Sakong. 2010. “Unhealthy Travelers Present Challenges to Sustainable Primate Ecotourism.” Travel Medicine and Infectious Disease 8 (3): 169–175. doi: 10.1016/j.tmaid.2010.03.004.
Nekaris, Anne-Isola, Nicola Campbell, Tim G. Coggins, E. Johanna Rode, and Vincent Nijman, 2013. “Tickled to Death: Analysing Public Perceptions of ‘Cute’ Videos of Threatened Species (Slow Loris – Nycticebus spp.) on Web 2.0 Sites.” PLoS ONE 8(7): e69215. doi: 10.1371/journal.pone.0069215.
Noss, Andrew J. 1998. “The Impacts of Cable Snare Hunting on Wildlife Populations in the Forests of the Central African Republic.” Conservation Biology 12 (2): 390–398.
Nunn, Charles L., and Sonia Altizer. 2006. Infectious Diseases in Primates. Oxford, UK: Oxford University Press.
Nunn, Charles L., and Thomas R. Gillespie. 2016. “Infectious Disease and Primate Conservation.” In An Introduction to Primate Conservation, edited by Serge A. Wich and Andrew J. Marshall, 157–174. Oxford, UK: Oxford University Press.
Osei-Tutu, Paul. 2017. “Taboos as Informal Institutions of Local Resource Management in Ghana: Why They Are Complied With or Not.” Forest Policy and Economics 85 (1): 114–123. doi: 10.1016/j.forpol.2017.09.009.
Pavelka, Mary S. M., and Colin A. Chapman. 2006. “Population Structure of Black Howlers (Alouatta pigra) in Southern Belize and Responses Hurricane Iris.” In New Perspectives on the Study of Mesoamerican Primates: Distribution, Ecology, Behavior, and Conservation, edited by Alejandro Estrada, Paul A. Garber, Mary S. M. Pavelka, and LeAndra Luecke, 143–163. New York: Springer.
Pearce, John, and Gianna Moscardo. 2015. “Social Representations of Tourist Selfies: New Challenges for Sustainable Tourism.” In BEST EN Think Tank X, The Environment-People Nexus in Sustainable Tourism: Finding the Balance, 59-73. BEST EN Think Tank XV, 17-21 June 2015, Skukuza, Mpumalanga, South Africa.
Pebsworth, Paula A., and Marni LaFleur. 2014. “Advancing Primate Research and Conservation through the Use of Camera Traps: Introduction to the Special Issue.” International Journal of Primatology 35 (5): 825–840. doi: 10.1007/s10764-014-9802-4.
Peterson, Jeffrey V. 2017. “Primates in World Religions (Buddhism, Christianity, Hinduism, Islam).” In The International Encyclopedia of Primatology, edited by Agustín Fuentes,1171–1177. Hoboken, NJ: John Wiley and Sons. doi: 10.1002/9781119179313.wbprim0122.
Peterson, Jeffrey V., and Erin P. Riley. 2017. “Sacred Monkeys? An Ethnographic Perspective on Macaque Sacredness in Balinese Hinduism.” In Ethnoprimatology: A Practical Guide to Research at the Human-Nonhuman Primate Interface, edited by Kerry M. Dore, Erin P. Riley, and Agustín Fuentes, 206-218. Cambridge, UK: Cambridge University Press.
Ratsimbazafy, J. 2006. “Diet Composition, Foraging, and Feeding Behavior in Relation to Habitat Disturbance: Implications for the Adaptability of Ruffed Lemurs (Varecia v. editorium) in Manombo Forest, Madagascar.” In Lemurs: Ecology and Adaptation, edited by L. Gould and M. L. Sauther, 403–422. New York: Springer.
Reibelt, L. M., and J. Nowack. 2015. “Editorial: Community-Based Conservation in Madagascar, the ‘Cure-All’ Solution? Madagascar Conservation & Development. 10 (1): 3–5. doi: 10.4314/mcd.v10i1.S1.
Rimbach, Rebecca, Andrés Link, Andrés Montes-Rojas, Anthony Di Fiore, Michael Heistermann, and Eckhard W. Heymann. 2014. “Behavioral and Physiological Responses to Fruit Availability of Spider Monkeys Ranging in a Small Forest Fragment.” American Journal of Primatology 76 (11): 1049–1061. doi: 10.1002/ajp.22292.
Robbins, Martha M., Markye Gray, Katie A. Fawcett, Felicia B. Nutter, Prosper Uwingeli, Innocent Mburanumwe, Edwin Kagoda, et al. 2011. “Extreme Conservation Leads to Recovery of the Virunga Mountain Gorillas.” PLoS ONE 6 (6): e19788. doi: 0.1371/journal.pone.0019788.
Roncal, Carla Mere, Mark Bowler, and Michael P. Gilmore. 2018. “The Ethnoprimatology of the Maijuna of the Peruvian Amazon and Implications for Primate Conservation.” Journal of Ethnobiology and Ethnomedicine 14 (19). doi: 10.1186/s13002-018-0207-x.
Rothman, Jessica M., Colin A. Chapman, Thomas T. Struhsaker, David Raubenheimer, Dennis Twinomugesha, and Peter G. Waterman. 2015. “Long-term Declines in Nutritional Quality of Tropical Leaves.” Ecology 96 (3): 873–878. doi: 10.1890/14-0391.1.
Russo, Sabrina E. 2017. “Seed Dispersal.” In The International Encyclopedia of Primatology, edited by Agustín Fuentes, 1265–1269. Hoboken, NJ: John Wiley and Sons.
Russo, Sabrina, and Colin Chapman. 2011. “Primate Seed Dispersal: Linking Behavioral Ecology with Forest Community Structure.” In Primates in Perspective, edited by Christina Campbell, Agustín Fuentes, Katherine McKinnon, Simon Bearder, and Rebecca Stumpf, 523–534. New York: Oxford University Press.
Rylands, Anthony B., and Russell A. Mittermeier. 2014. “Primate Taxonomy: Species and Conservation.” Evolutionary Anthropology 23 (1): 8–10. doi: 10.1002/evan.21387.
Scales, I. R. 2014. “The Future of Biodiversity Conservation and Environmental Management in Madagascar: Lessons from the Past and Challenges ahead.” In Conservation and Environmental Management in Madagascar, edited by I. R. Scales, 342–360. London: Routledge.
Schaffner, Colleen M., Luisa Rebecchini, Gabriel Ramos-Fernandez, Laura G. Vick, and Fillipo Aureli. 2012. “Spider Monkeys (Ateles geoffroyi yucatenensis) Cope with the Negative Consequences of Hurricanes through Changes in Diet, Activity Budget, and Fission–Fusion Dynamics.” International Journal of Primatology 33 (4): 922–936. doi: 10.1007/s10764-012-9621-4.
Schloss, Carrie A., Tristan A. Nuñez, and Joshua J. Lawler. 2012. “Dispersal Will Limit Ability of Mammals to Track Climate Change in the Western Hemisphere.” PNAS 109 (22): 8606–8611. doi: 10.1073/pnas.1116791109.
Schwitzer, Christoph, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis, and Alison Cotton, eds. 2017.“Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018.” Arlington, VA: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society.
Sechrest, W., Thomas M. Brooks, Gustavo A. B. da Fonseca, William R. Konstant, Russel A. Mittermeier, Andy Purvis, Anthony B. Rylands, and John L. Gittleman. 2002. “Hot Spots and the Conservation of Evolutionary History.” Proceedings of the National Academy of Sciences 99 (4): 2067–2071. doi: 10.1073/pnas.251680798.
Shanee, Noga, A. Patricia Mendoza, and Sam Shanee. 2017. “Diagnostic Overview of the Illegal Trade in Primates and Law Enforcement in Peru.” American Journal of Primatology 79 (11): 1–12. doi: 10.1002/ajp.22516.
Shanee, N., S. Shanee, and A. M. Maldonado. 2007. “Conservation Assessment and Planning for the Yellow-Tailed Woolly Monkey (Oreonax flavicauda) in Peru.” Wildlife Biology in Practice. 3 (2): 73–82. doi: 10.2461/wbp.2007.3.9.
Sicotte, Pascale. 2017. “Social Taboos.” In The International Encyclopedia of Primatology, edited by Agustín Fuentes, 1319–1321. Hoboken, NJ: John Wiley and Sons. doi: 10.1002/9781119179313.wbprim0117.
Southwick, Charles H., and M. Farooq Siddiqi. 1988. “Partial Recovery and a New Population Estimate of Rhesus Monkey Populations in India.” American Journal of Primatology 16 (3): 187–197.
Spenceley, Anna, Straton Habyalimana, Ritah Tusabe, and Donnah Mariza. 2010. “Benefits to the Poor from Gorilla Tourism in Rwanda.” Development Southern Africa 27 (5): 647–662. doi: 10.1080/0376835X.2010.522828.
Sponsel, Leslie E. 1997. “The Human Niche in Amazonia: Explorations in Ethnoprimatology.” In New World Primates: Ecology, Evolution, and Behavior, edited by W. Kinzey, 143–165. New York: Aldine de Gruyter.
Srivastava, Arun, Jayanta Das, Jihosuo Biswas, Pranab Buzarbarua, Prabal Sarkar, Irwin S. Bernstein, and Surendra Mal Mohnot. 2001. “Primate Population Decline in Response to Habitat Loss: Borajan Reserve Forest of Assam, India.” Primates 42 (4): 401–406. doi: 10.1007/BF02629631.
Steinweg, Tim, Barbara Kuepper, and Gabriel Thoumi. 2016. Economic Drivers of Deforestation. Washington, DC: Chain Reaction Research. http://chainreactionresearch.com/wp-content/uploads/2016/08/economic-drivers-of-deforestation-crr-160803-final1.pdf
Strier, Karen B. 1994. “Myth of the Typical Primate.” American Journal of Physical Anthropology 37 (S19): 233–271. doi: 10.1002/ajpa.13303700609.
———. 2003. “Primatology Comes of Age: 2002 AAPA Luncheon Address.” Yearbook of Physical Anthropology 46: 2–13.
———. 2010. “ Long-term Field Studies: Positive Impacts and Unintended Consequences.” American Journal of Primatology 72 (9): 772–778. doi: 10.1002/ajp.20830.
———. 2011a. “Conservation.” In Primates in Perspective, edited by Christina Campbell, Agustín Fuentes, Katherine C. MacKinnon, Simon K. Bearder, and Rebecca M. Stumpf, 664–675. New York: Oxford University Press.
———. 2011b. “Why Anthropology Needs Primatology.” General Anthropology 18 (1): 1–8.
———. 2017. Primate Behavioral Ecology: Fifth Edition. New York: Routledge.
———. 2018a. “Primate Social Behavior.” American Journal of Physical Anthropology 165 (4): 801–812.
———. 2018b. “What Climate Change Means for Primates and Primatology.” Paper presented at the 87th Annual Meeting of American Association of Physical Anthropologists, Austin, Texas, April 11–14, 2018.
Strier, Karen B. and Jean Philippe Boubli. 2006. “A History of Long-term Research and Conservation of Northern Muriquis (Brachyteles hypoxanthus) at the Estação Biológica de Caratinga/RPPN-FMA.” Primate Conservation 20: 53–63. doi: 10.1896/0898-6207.20.1.53.
Strier, Karen B. and Sérgio L. Mendes. 2012. “The Northern Muriqui (Brachyteles hypoxanthus): Lessons on Behavioral Plasticity and Population Dynamics from a Critically Endangered Species.” In Long-term Field Studies of Primates, edited by Peter M. Kappeler and David P. Watts, 125–140. Berlin, Heidelberg: Springer.
Strier, Karen B., Carla B. Possamai, Fernanda P. Tabacow, Alcides Pissinatti, Andre M. Lanna, Fabiano Rodrigues de Melo, Leandro Moreira, et al. 2017. “Demographic Monitoring of Wild Muriqui Populations: Criteria for Defining Priority Areas and Monitoring Intensity.” PLoS ONE: 12 (12): e0188922. doi: 10.1371/journal.pone.0188922.
Strum, Shirley C., and Linda M. Fedigan. 1999. “Theory, Method, Gender, and Culture: What Changed Our Views of Primate Society.” In The New Physical Anthropology: Science, Humanism, and Critical Reflection, edited by S. C. Strum, D. G. Lindburg, and D. A. Hamburg, 67–105. New Jersey: Prentice Hall.
Tarara, R., M. A. Suleman, R. Sapolsky, M. J. Wabomba, and J. G. Else. 1985. “Tuberculosis in Wild Olive Baboons (Papio cynocephalus anubis) in Kenya.” Journal of Wildlife Diseases 21 (2): 137–140. doi: 10.7589/0090-3558-21.2.137.
Teaford, Mark F., and Ordean J. Oyen. 1989. “In Vivo and In Vitro Turnover in Dental Microwear.” American Journal of Physical Anthropology 80 (4): 447–600. doi: 10.1002/ajpa.1330800405.
Terborgh, J. 1983. Five New World Primates: A Study in Comparative Ecology. Princeton: Princeton University Press.
Tumusiime, David Mwesigye, Gerald Eilu, Mnason Tweheyo, Mnason Tweheyo, and Fred Babweteera. 2010. “Wildlife Snaring in Budongo Forest Reserve, Uganda.” Human Dimensions of Wildlife 15 (2): 129–144. doi: 10.1080/10871200903493899.
Tyukavina, Alexandra, Matthew C. Hansen, Peter V. Potapov, Stephen V. Stehman, Kevin Smith-Rodriguez, Chima Okpa, and Ricardo Aguilar. 2017. “Types and Rates of Forest Disturbance in Brazilian Legal Amazon, 2000–2013.” Science Advances 3 (4): 1–16. doi: 10.1126/sciadv.1601047.
UN Population Division. 2017. “World Population Prospects: The 2017 Revision.” Accessed January 2, 2019. https://esa.un.org/unpd/wpp/publications/files/wpp2017_keyfindings.pdf.
Ungar, Peter S., and Matt Sponheimer. 2011. “The Diets of Early Hominins.” Science 334 (6053): 190–193.
Voigt, Maria, Serge A Wich, Marc Ancrenaz, Erik Meijaard, Nicola Abram, Graham L. Banes, Gail Campbell-Smith, et al. 2018. “Global Demand for Natural Resources Eliminated More Than 100,000 Bornean Orangutans.” Current Biology 28 (5): 761–769. doi: 10.1016/j.cub.2018.01.053.
Wallis, Janette, and D. Rick Lee. 1999. “Primate Conservation: The Prevention of Disease Transmission.” International Journal of Primatology 20 (6): 803–826. doi: 10.1023/A:1020879700286.
Wang, Chengliang, Xiaowei Wang, Xiaoguang Qi, Songtao Guo, Haitao Zhao, Wei Wei, and Baoguo Li. 2015. “Influence of Human Activities on the Historical and Current Distribution of Sichuan Snub-Nosed Monkeys in the Qinling Mountains, China.” Folia Primatologica 85 (6): 343–357.
Washburn, Sherwood L. 1951. “Section of Anthropology: The New Physical Anthropology.” Transactions of the New York Academy of Sciences 13 (7): 298–304.
———.1973. “The Promise of Primatology.” American Journal of Physical Anthropology 38 (2): 177–182.
Wheatley, Bruce P. 1999. The Sacred Monkeys of Bali. New York: Waveland.
Wiederholt, Ruscena, and Eric Post. 2010. “Tropical Warming and the Dynamics of Endangered Primates.” Biology Letters. 6 (2): 257–260. doi: 10.1098/rsbl.2009.0710.
Williams, J. M., E. V. Lonsdorf, M. L. Wilson, J. Schumacher-Stankey, J. Goodall, and A. E. Pusey. 2008. “Causes of Death in the Kasekela Chimpanzees of Gombe National Park, Tanzania.” American Journal of Primatology 70 (8): 766–777. doi: 10.1002/ajp.20573.
Wright, Patricia. 1999. “Lemur Traits and Madagascar Ecology: Coping with an Island Environment.” Yearbook of Physical Anthropology 42: 31–72. doi: 10.1002/(SICI)1096-8644(1999)110:29+<31::AID-AJPA3>3.0.CO;2-0.
Wright, S. Joseph. 2005. “Tropical Forests in a Changing Environment.” Trends in Ecology and Evolution 20 (10): 553–560. doi: 10.1016/j.tree.2005.07.009.
Yersin, Harmony, Caroline Asiimwe, Maarten J. Voordouw, and Klaus Zuberbühler. 2017. “Impact of Snare Injuries on Parasite Prevalence in Wild Chimpanzees (Pan Troglodytes).” International Journal of Primatology 38 (1): 21–30. doi: 10.1007/s10764-016-9941-x.
Zhao, Maosheng, and Steven W. Running. 2010. “Drought-Induced Reduction in Global Terrestrial Net Primary Production from 2000 through 2009.” 2010. Science 329 (5994): 940–943. doi: 10.1126/science.1192666.
Zimmerman, Julie K. H., and Alan P. Covich. 2007. “Damage and Recovery of Riparian Sierra Palms after Hurricane Georges: Influence of Topography and Biotic Characteristics.” Biotropica 39 (1): 43–49. doi: 10.1111/j.1744-7429.2006.00237.x.
Acknowledgments
We are grateful to the University of Wisconsin-Madison for the various sources of funding that enabled us to write this Appendix, including Teaching Assistantships from the departments of Integrative Biology (to MPD and JBK) and Anthropology (to RJE and AJH), an Advanced Opportunity Fellowship (to IEA), and a Vilas Research Professorship (to KBS). We are grateful to our new lab member, Irene Duch Latorre, for her photograph and helpful additions as well as to Kadie Callingham for the use of her photograph. We thank the editors of this volume for inviting our contribution and for the helpful comments that they and an anonymous reviewer provided.
Figure Attributions
Figure B.1 World population by region by Our World in Data [Source HYDE (2016) & UN (2019)] accessed August 5, 2019 is used under a CC BY 4.0 License.
Figure B.2 Global primate species richness, distributions, and the percentage of species threatened and with declining populations (Figure 1) by Estrada et al. [Original: 2017 “Impending Extinction Crisis of the World’s Primates: Why Primates Matter.” Science Advances 3 (229): 1–16. doi:10.1126/sciadv.1600946] is used under a CC BY 4.0 License.
Figure B.3 Mountain Gorilla Bwindi by Rod Waddington is used under a CC BY-SA 2.0 License.
Figure B.4 A female northern muriqui (Brachyteles hypoxanthus) with infant at the Feliciano Miguel Abdala Private Natural Heritage Reserve outside of Caratinga, Brazil by Amanda J. Hardie, courtesy of Projeto Muriqui de Caratinga, is used by permission and available here under a CC BY-NC 4.0 License.
Figure B.5 International Union for Conservation of Nature (IUCN) Criteria for Threatened Taxa by Mary P. Dinsmore et al., updated from Strier 2011, with data simplified and condensed from IUCN Species Survival Commission (2012), is under a CC BY-NC 4.0 License.
Figure B.6 Bornean Orangutan Wide Face by Eric Kilby is used under a CC BY-SA License.
Figure B.7 A female gelada (Theropithecus gelada) with a snare around its neck in central Ethiopia by Kadie Callingham is used by permission and available here under a CC BY-NC 4.0 License.
Figure B.8 Cattle graze in papaya plantation, once forested land, in Montagne des Français, Madagascar by Mary P. Dinsmore is under a CC BY-NC 4.0 License.
Figure B.9 Forest cleared for cattle-ranching in the province of Manabí, Ecuador by Irene Duch-Latorre, courtesy of Proyecto Washu, is used by permission and available here under a CC BY-NC 4.0 License.
Figure B.10 An industrial-sized truck with charcoal leaves Montagne des Français region, Madagascar by Mary P. Dinsmore is under a CC BY-NC 4.0 License.
Figure B.11 Old-growth tree uprooted after Cyclone Enawo, Madagascar by Mary P. Dinsmore is under a CC BY-NC 4.0 License.
Figure B.12 A northern sportive lemur (Lepilemur septentrionalis), Montagne des Français, Madagascar by Mary P. Dinsmore is under a CC BY-NC 4.0 License.
Figure B.13 A model of the extinction vortex drawn by Karen B. Strier (AAPA 87th Annual Meeting, 11-14 April 2018), adapted from Gilpin and Soulé (1986), is available here under a CC BY-NC 4.0 License.
Figure B.14 Fecal matter with seeds from the large-bodied northern muriqui (Brachyteles hypoxanthus) by Amanda J. Hardie, courtesy of Projeto Muriqui de Caratinga, is used by permission and available here under a CC BY-NC 4.0 License.
Figure B.15 Tourists feeding monkeys ATR P1180869 by T. R. Shankar Raman is used under a CC BY-SA 4.0 License.
Figure B.16 Students in the canopy at El Zota field station, Costa Rica, by Mary P. Dinsmore, courtesy of Broadreach Global Summer Adventures, Inc. is used by permission and available here under a CC BY-NC 4.0 License.
