Private: Main Body
6 Primate Ecology and Behavior
Karin Enstam Jaffe, Ph.D., Sonoma State University
Learning Objectives
- Describe the behavioral variation that exists within the Primate Order and how primate behavior and morphology are influenced by diet, predation, and other ecological factors.
- Explain why primates live in groups.
- Distinguish primate social systems from mating systems.
- Contrast male and female reproductive and parental investment strategies.
- Describe the ways in which primates communicate.
- Evaluate the evidence for primate cultural variation.


Nonhuman primates (from now on simply referred to as “primates”) are our closest living relatives, and their behavior is often strikingly similar to our own. If you’ve ever seen a female monkey at your local zoo cooing over her newborn baby (Figure 6.1a) or watched a video of a tufted capuchin monkey using rocks as a hammer and anvil to crack open a nut to access the edible kernel inside (Figure 6.1b), then you know how interesting they can be.

I have been fascinated by primates since I was a young child. In the summer of 1996, I went with my dissertation advisor, Dr. Lynne A. Isbell, to her field site in Laikipia, Kenya (Figure 6.2) with the intention of studying the play behavior of juvenile patas monkeys. One day we were following our patas study group when several females and juveniles began giving high-pitched “nyow” alarm calls. I was awestruck as I watched the entire group take off at breakneck speed. Patas monkeys are, after all, the fastest primate, capable of running 20 miles per hour for short distances. It did not even occur to me that they had sounded an alarm and then run away from something—until my advisor pointed to the lioness hidden in the grass at the base of a tree. (We slowly backed away and got in our car.) My research interests changed in that moment: I wanted to study primate antipredator behavior, the strategies primates use to escape from predators. I would spend two years at that same field site collecting data on anti-predator behavior of patas monkeys and vervets, two closely related species who occupy different habitats. Patas monkeys (Figure 6.3a) live far from rivers, in habitats composed of short trees spaced far apart (Figure 6.3b). These trees have little to no overlapping canopy, so climbing one to escape a lion in pursuit can result in a literal dead end. In contrast, vervets (Figure 6.4a) spend most of their time along rivers, with access to tall trees with overlapping canopies (Figure 6.4b) that provide good escape routes from terrestrial predators. But they also venture into patas habitats, the short trees with canopies that do not overlap. I wanted to know: How would the structure of these habitats affect the responses of vervets and patas monkeys to alarm calls that signal the approach of a terrestrial predator like a lion? Not surprisingly, when vervets are near the river, they climb the tall trees to seek refuge from such predators. But not patas monkeys. These “cheetahs of the primate world” are more likely to take off running (as I had seen them do that summer), even bypassing nearby trees. Their physical adaptations for speed, like their long legs, combined with the lack of arboreal escape routes, makes fleeing on the ground their best option. But what do vervets do when they are away from the river and the safety of their tall trees? Is their behavior “hard-wired” so that their response to an alarm call is the same, regardless of the habitat? Or do they assess key aspects of their habitat, like tree height and canopy cover, and alter their behavior? Although they cannot run as fast, when they hear an alarm call they run back toward the river, by-passing the short trees, just like the patas do (Enstam and Isbell 2002). The implication is clear: these monkeys, our close relatives, with their highly developed intelligence and ability to learn, do assess key features of their habitat and use this information to alter their behavior and maximize their chance of escape.


Figure 6.3a and 6.3b A female patas monkey with infant (left). A patas habitat in Laikipia, Kenya (right).


Figure 6.4a and 6.4b A female vervet (left). A vervet habitat in Laikipia, Kenya (right).

The branch of science that focuses on the study of primate behavior is called , and people, like myself, who study primates (Figure 6.5) are called . Primatologists come from many different disciplines and study primate behavior for different reasons. Biologists study primates as examples of evolutionary theories like natural selection or parental investment. Primate intelligence is of interest to psychologists who want to learn more about the underlying cognitive principles involved in deceptive or cooperative behavior and to linguists interested in the principles of communication and language. Ecologists studying conservation issues examine how primates are affected by deforestation, poaching, or illegal animal trade. Biological anthropologists, like myself, who study primates are interested in their social complexity and ecological and behavioral variation. Because both humans and most nonhuman primates live in groups, biological anthropologists study primates to better understand the evolution of social behavior and its costs and benefits. Because primates are our closest living relatives, we study them to gain insights into how our human ancestors may have behaved as well as to better understand our own behavior.
ECOLOGY
The more than 600 species and subspecies of living primates are highly diverse in their dietary preferences and the habitats they occupy. These aspects of primate ecology have significant impacts on every part of a primate’s life, including their morphology, physiology, and body size as well as their interactions with other individuals inside and outside their social group. They even play a role in determining whether a primate lives in a group or is and lives alone. A primate’s habitat determines the food to which they have access and the community of other species with whom they interact, including predators.
Primate Diets
Diet may be the most important variable influencing variation in primate morphology, behavior, and ecology, and primate diets are highly varied. Some primatologists separate , the act of finding and handling food, from , the act of consuming food, while others combine these into one category. Most primates are who ingest a variety of foods in order to obtain appropriate levels of protein, carbohydrates, fats, and fluids, but one type of food often makes up the majority of each species’ diet. Because you learned about the dental and digestive adaptations experienced by (who feed primarily on fruit), (whose diet consists mostly of leaves), and (who eat mainly insects) in Chapter 5, we will not discuss them here. Instead, we will focus on the relationship between diet and body size and the variation in food (how much is available in a given area) and (how it is spread out).
Body Size and Diet


As you learned in Chapter 5, insects are a high-quality food. Full of easily digestible protein and high in calories, insects are an excellent source of nutrients, meeting most of a primate’s dietary needs. Although all primates will eat insects if they come upon them, those species that rely most heavily on insects tend to be the smallest. If insects are such a high-quality food, why aren’t all primates insectivores? The answer is that larger primates simply cannot capture and consume enough insects every day to survive. This is because the (BMR), or the rate at which energy is used to maintain the body while at rest, increases more slowly than body size. The result? Heavier animals must consume absolutely more food, but they have a slower metabolism so they need fewer calories per unit of body weight. Because of their small size (less than 150 g), tarsiers do not need to consume large amounts of food each day, but their high metabolic rate means they convert food into energy very quickly. This is only possible by consuming food that is easily digestible, like insects. It does not matter to a tarsier that a grasshopper only weighs 300 mg, because the tarsier itself is so small that one grasshopper is a good-size meal (Figure 6.6a). However, an adult male gorilla, who may weigh up to 200 kilograms, cannot possibly consume enough insects to meet its caloric needs. And it does not need to. Because of their large body size, gorillas have a much lower metabolic rate than tarsiers, so they can consume low-quality food, like leaves, and take their time digesting it, so long as they get enough (Figure 6.6b). Fortunately for gorillas, leaves are plentiful, as we will see in the next section. Most medium-size primates are highly frugivorous. Whether they supplement their high-fruit diet with insects or leaves also depends on their size. Smaller frugivores tend to supplement with insects, while larger frugivores tend to supplement with leaves.
Food Abundance and Distribution


Nutrients (see Chapter 5) and food quality are not the only dietary considerations primates must make. They must also ensure that they consume more calories than they burn. The abundance and distribution of food affect energy expenditure and calorie intake because they determine how far animals must travel in search of food and how much they must compete to obtain it. Abundance refers to how much food is available in a given area while distribution refers to how food is spread out. Food abundance is either plentiful (Figure 6.7a) or scarce (Figure 6.7b). Food is distributed in one of three ways: uniformly (Figure 6.8a), in clumps (Figure 6.8b), or randomly (Figure 6.8c). In general, higher-quality foods, like fruit and insects, are less abundant and have patchier distributions than lower-quality foods, like leaves. In a rainforest, like the Amazon, every tree has leaves, so they are abundant and uniformly distributed (Figure 6.9a). Folivores do not have to travel very far to find food so they do not burn many calories searching for food. In comparison to leaves, fruit is scarce and clumped (Figure 6.9b). Because not every tree contains fruit, it is less plentiful than leaves. In addition, in a rainforest, a single tree with fruit may be surrounded by many trees without fruit. To a frugivore, this one fruit tree is a clump of fruit. Frugivores who do not eat leaves must travel farther distances in search of food because they can only feed in some trees (i.e., those producing fruit). Frugivores burn more calories searching for food than folivores, so it is a good thing that fruit is such a high-quality food. Lastly, insects are scarce, and due to their mobile nature, most are randomly distributed (Figure 6.9c). This combination makes it impossible for larger primates to rely on insects for a significant part of their diet.






It is important to remember that species preferences for specific types of food may cause it to exist in abundance or distribution that is different than the general patterns we’ve discussed here. For example, for folivores who prefer young (i.e., immature) leaves, their food supply is patchier and less abundant than it appears to a researcher looking at the lush green carpet of the Amazon forest because only some leaves are immature at any point in time. During fruiting season, when many trees are producing fruit, fruit may be temporarily abundant and less clumped. Similarly, some insects, like termites in a termite mound (Figure 6.10), are found in clumps, similar to the way a single fruit tree is a “clump” of fruit surrounded by trees with no fruit.
Competition for Food

When a resource that is important for survival or reproduction is scarce, individuals will compete to obtain that resource. This is a central tenet of Charles Darwin’s theory of evolution by natural selection (see Chapter 2). Because female primates (like all mammals) devote a lot of energy to offspring production and care (discussed in detail in the “Parental Investment” section of this chapter), especially while pregnant and nursing, they compete for access to food, so long as the food is worth competing for. Competition between primates takes two forms: Individuals engage in direct competition (e.g., fighting) over resources that are large and worth defending (fruit is a good example of a food resource over which primates will fight) or individuals engage in indirect competition (e.g., eating food before another individual gets to it), which occurs when a resource is small or not worth defending. Primates often engage in indirect competition for insects, like grasshoppers, that are eaten quickly (Figure 6.6a). Primates may engage in direct and/or indirect competition with members of their own group or with members of other groups.
Effects of Food Abundance and Distribution on Interactions Between and Within Groups
The amount, or abundance, of food determines the nature of competition between different groups (Isbell 1991). Between-group competition is seen in terms of changes to home range size and nature of interactions between groups. A group’s is the area over which the group moves in search of food. Groups that defend the boundary of their home range are said to occupy a . Consider a patch of forest covered in leaves (e.g., Figure 6.9a). If you are a folivore, every tree is a dinner table. When your group size increases, your home range does not expand because there is more than enough food for everyone, and it is a waste of energy to travel farther than you need to. And as long as your group does not expand its home range, you will not encroach on the trees of neighboring groups. This keeps competitive interactions between groups of folivores to a minimum. Now imagine you are a frugivore. Unlike leaves, not every tree has fruit on it. Maybe only two or three trees are in fruit at any given time, so fruit is scarce (Figure 6.9b). Thus, if your group size increases, it is likely that the few fruit trees available in your current home range will not be able to feed everyone. If that’s the case, then your group will need to expand its home range in search of additional fruit trees, which are in the home ranges of neighboring groups. Home-range expansion is also often accompanied by fighting between groups as members attempt to keep intruders away from valuable, scarce food resources.
While food abundance determines interactions between groups, food distribution determines the interactions between individuals within a group (Isbell 1991). Competition within a group is marked by changes in day-range length and the presence of dominance hierarchies. measures the distance a group must travel in a single day in search of food. A reflects the place of each individual in the group in comparison to others. An individual’s place in the hierarchy, or “rank,” determines their priority of access to resources. If food is evenly distributed (as with leaves; Figure 6.9a), individuals can spread out while feeding so that their day-range length does not increase when their group size increases. Likewise, because leaves are “everywhere,” there is little benefit to females engaging in interactions that determine “priority of access” to resources, so dominance hierarchies are uncommon among folivores. However, if food is clumped (as with fruit; Figure 6.9b), individuals in groups must feed in more cohesive units (i.e., all in one fruit tree). When group size increases, the group must travel farther each day in order to visit enough fruit trees to feed all group members. Likewise, when food is clumped, individuals have the opportunity to monopolize it and keep others from feeding. Under such circumstances, females benefit from competing with one another for “priority of access” to the resource, and dominance hierarchies result.
The fact that food abundance and food distribution vary independently helps us understand the complex nature of between-group and within-group interactions (Isbell 1991). For example, both olive baboons and patas monkeys feed on scarce resources, and both species engage in competition with other groups and expand their home-range size when food is in short supply. But olive baboons’ food is clumped while patas monkeys’ is dispersed, so the interactions within groups are very different. Female baboons have a strong dominance hierarchy, and the distance they travel each day increases with group size. Patas monkeys have a weak dominance hierarchy, and when group size increases, individuals spread out while feeding and daily travel distance does not increase.
Community Ecology


In addition to interactions with other members of their own group and other groups of (members of the same species), primates are members of broader ecological communities composed of other species, including other primates, predators, and even humans. When two species (or populations) occupy the same geographic area, they are . The patas monkeys and vervets that I studied in Kenya, along with olive baboons and Senegal bush babies, are sympatric and form a (Figure 6.11a–d). However, vervets (Figure 6.11b) and muriquis of Brazil (Figure 6.12) are , meaning their geographic ranges do not overlap. Some habitats support highly diverse primate communities consisting of 10 or more species (Figure 6.13). How can so many species of primate occupy the same area and avoid competition? Sympatric species do sometimes compete with each other.


Observations of one species displacing another at a food site is a sign of competition between the two species. When this happens, usually it is the large-bodied species that supplants the small-bodied species. The exception is when the small-bodied species significantly outnumbers the larger-bodied one. The states that two species that compete for the exact same resources cannot coexist. This means that two species cannot occupy the same —cannot seek to meet their needs for food and shelter in the exact same way. Because tropical rainforests are highly variable, with many habitats and many sources of food and shelter, there are many different niches for multiple species

to exploit, and large primate communities result (Figure 6.13). In non-rainforest habitats, like Kenya’s open woodland, which is home to four species (Figure 6.11a–d), there are fewer niches for multiple species to occupy. Regardless of habitat type, sympatric species avoid competition through (using the environment differently). Niche partitioning includes differences in diet, ranging behavior, and habitat use. In Laikipia, Kenya, bush babies reduce competition with vervets by feeding more heavily on insects. They further reduce competition by being nocturnal while vervets are diurnal. Even though bush babies (Figure 6.11d) and vervets (Figure 6.11b) do sometimes eat the same food, since they eat at different times of day they rarely, if ever, interact.
|
Site |
Primate Community |
|
Krau Game Reserve, Malaysia |
white-handed gibbon, siamang, dusky leaf monkey, mitered leaf monkey, long-tailed macaque, pigtail macaque, slow loris |
|
Manu National Park, Peru |
black spider monkey, red howler monkey, brown capuchin, white-fronted capuchin, South American squirrel monkey, owl monkey, dusky titi monkey, common woolly monkey, monk saki monkey, Goeldi’s marmoset, emperor tamarin, saddleback tamarin, pygmy marmoset |
|
Beza Mahafaly Reserve, Madagascar |
ring-tailed lemur, Verreaux’s sifaka, white-footed sportive lemur, reddish-gray mouse lemur |
|
Kibale National Park, Uganda |
Ugandan mangabey, L’Hoest’s monkey, Ugandan red colobus monkey, vervet monkey, olive baboon, blue monkey, grey-cheeked mangabey, potto, galago, black and white colobus monkey, chimpanzee |
Figure 6.13 Examples of primate communities.
Predation


An important aspect of primate communities is the predators that also occupy them. As discussed in the “Primate Diets” section, all primates incorporate some insects into their diet, and so they may be themselves considered predators in this respect. In this section, we will limit our discussion to predation of and by (animals with an internal spinal column or backbone). Many primates incorporate some vertebrate prey into their diet. Often, predation by primates is opportunistic, occurring because the prey happen to be in the right place at the right time. I’ve observed vervets opportunistically killing lizards by smashing them against a rock or tree trunk and eating them (much as this lion-tailed macaque has done; Figure 6.14a). In some parts of their range (including Gombe Stream National Park and Mahale Mountain National Park, both in Tanzania), chimpanzees are described as opportunistic hunters, with the vast majority of hunts occurring after a chance encounter with prey (Figure 6.14b; Boesch and Boesch 1989). Other primates are more deliberate predators, and some even work together to increase their chances of success. Cooperative hunting has been observed in white-faced capuchins and some chimpanzee populations. White-faced capuchins hunt more often during the dry season, when other food is scarce, and sometimes work together to chase, surround, and capture small mammals like young squirrels or coatis (Fedigan 1990). The chimpanzees of Taï National Park in Côte d’Ivoire take deliberate, cooperative hunting to the next level. Unlike their Tanzanian counterparts, they form hunting parties to search for red colobus monkeys and, once located, anticipate the prey’s movement and coordinate with other hunters to drive, isolate, and capture prey (Boesch 2002). Since hunting-party size correlates with hunting success, it is not surprising that sharing the spoils of a successful hunt is more common in Taï chimpanzees who rely on others for success (Boesch and Boesch 1989).

All primates are susceptible to predation by mammalian (animals whose diet consists primarily of animal tissue) (Figures 6.15a–d), birds of prey (Figures 6.15e-f), and/or reptiles (Figure 6.15g), although the specific predators differ based on geography and primate body size. Smaller primates fall prey to a wider range of predators than larger primates, and some habitats contain a greater diversity of predators. Primates use a variety of anti-predator tactics to avoid and/or escape predation. Perhaps the best way to avoid predation is to avoid being detected by predators in the first place, and some primates use to great effect.

Nocturnal primates are often small and solitary or live in very small groups. If you are already hard to see because you are active at night, moving quietly in small groups is a good strategy to avoid detection by predators. The slow loris of Southeast Asia exemplifies this strategy (Figure 6.16). Nocturnal and solitary, the slow loris moves slowly (as its name suggests) and quietly as its primary strategy to avoid predation (Wiens and Zitzmann 2003). If detected, however, the slow loris will attempt to escape by releasing its grip and falling off the branch or biting in defense.


Interestingly, the slow loris is the only venomous primate. The venom is formed when the slow loris combines oil from a gland on its arm with its saliva (Nekaris et al. 2013). It can either apply the venom to its head for protection or store it in the mouth to deliver through a bite. Slow loris bites are painful and take a long time to heal. In extreme cases, individuals who are bitten may go into shock and die. It is not as easy for diurnal primates to avoid detection by predators, and most (but not all) diurnal primates, like Hanuman langurs, have larger body sizes and live in groups (Figure 6.17). Indeed, anti-predator behavior, including vigilance, alarm calling, and mobbing, may be one of the primary benefits primates get from living in groups; we will discuss these behaviors in a later section, entitled “Why do Primates Live in Groups?”





SPECIAL TOPIC: PRIMATE CONSERVATION


There are over 600 species and subspecies of primates on the planet today, and almost half of them live under the threat of extinction. While there are many threats to primates, habitat destruction and hunting are the leading causes of population decline (Figure 6.18a–b). Primate populations have withstood small-scale forest clearing and low levels of hunting by local human groups for hundreds of years. However, the recent, intense pressure of expanding human populations on many primate habitats is resulting in rapid population declines for many species. The majority of primates live in tropical habitats, and the loss of tropical forest, whether due to logging or farming, is the single greatest factor contributing to the decline of primate populations across the planet. Between 1973 and 2010, almost 100,000 km2 of orangutan habitat was cleared for palm oil plantations in Borneo (Figure 6.18a). During this same time, the orangutan population decreased from almost 300,000 to 100,000, an average loss of more than 5,000 orangutans every year. As of 2017, that number may be as low as 60,000 (Schwitzer et al. 2017). If this rate of loss is not curtailed, the Bornean orangutan will go extinct in less than 15 years. Hunting, whether for bushmeat (Figure 6.18b), trophies, or the pet trade, has had devastating effects on many primate populations. Even though Grauer’s gorillas are legally protected, they are highly prized for bushmeat because they are relatively easy to track and shoot, and their large body size yields significant amounts of meat. Survey work has revealed that the Grauer gorilla population has declined significantly since the 1990s, due almost entirely to illegal hunting. The gorilla population in Kahuzi-Biega National Park, in Democratic Republic of Congo (DRC), is estimated to have declined 87% since 1994 (Schwitzer et al. 2017).
As consumers and concerned citizens, all of us are learning how to use our wallets to combat habitat and species loss. We do not buy palm oil or products made with palm oil in an effort to save orangutans. We donate to conservation organizations doing important on-the-ground work in Democratic Republic of Congo and other conservation hot-spots. We educate ourselves as well as our friends, families, and communities about the plight of endangered primates. Primatologists, too, contribute to conservation efforts. No longer is primatology research restricted to the “ivory tower” of academia. Current and future primatologists have the opportunity to affect real change in primate conservation (Chapman and Peres 2001). Whether understanding the mechanisms that determine species abundance, predicting the effects of human activity on species survival, documenting patterns of environmental change, understanding the effects of species removal in broader contexts, or evaluating different approaches to conservation, information gained from primate studies offers some of the best hope we have for a future that continues to include our closest living relatives. You can learn more about primate conservation in Appendix B.
SOCIALITY, RESIDENCY PATTERNS, AND DISPERSAL
The majority of mammal species are solitary, with individuals living alone, except for mothers and dependent offspring. However, most primate species live in groups. Primate groups vary in size, composition, and cohesiveness. Gibbons and siamangs of Southeast Asia and titi monkeys of South America form long-term pair bonds with groups consisting of an adult male and female with their dependent young. Ukaris of South America and ring-tailed lemurs of Madagascar both live in groups of up to 35 individuals containing multiple adult males and females, juveniles, and infants. Gorilla troops typically number between eight and 10 individuals, consisting of multiple females, juveniles, and infants but only one adult male, the silverback. Some primate groups (like gorillas, ukaris, and ring-tailed lemurs) are stable and cohesive over long periods, except for the of some individuals who leave the group. Others, like chimpanzees and spider monkeys, have more fluid social systems, called , where groups break up and reunite based on differences in food availability throughout the year. In this section, we’ll examine why primates live in groups and who stays, who goes, and why.
Why Do Primates Live in Groups?
Because group living is relatively unusual among mammals but quite common among primates, a central question for primatologists is: Why do primates live in groups? The answer is that primates live in groups when the benefits of feeding competition and/or predation avoidance exceed the costs.
Feeding Competition
As discussed in the previous section, when species feed on high-quality, scarce food (like fruit), larger groups mean there are more individuals competing for access to the resource. The result of this competition takes the form of dominance hierarchies and increased day-range length. A dominance hierarchy is the result of aggressive and submissive interactions, but once established, a dominance hierarchy functions to reduce levels of aggression because all individuals “know their place.” Female vervets illustrate the costs and benefits of different dominance ranks (Whitten 1983). Dominant (high-ranking) females spend more time feeding and eat more ripe fruit than subordinates (low-ranking), so they consume more nutrients. This affects their health and (an individual’s reproductive success relative to that of other individuals; Whitten 1983). Dominants weigh more, start reproducing earlier, and produce more offspring than subordinates do. So why do subordinate females remain in the group? The answer is that larger groups are more successful in competition with other groups. In a long-term study of vervets in Kenya’s Amboseli National Park, larger vervet groups had larger and better home ranges, which importantly included access to permanent sources of water. The result? Females in larger groups had shorter (the average length of time between one birth and the next) and higher average infant and female survival rates than the smallest group. In terms of competition for resources, the benefits of being a member of a larger vervet group (even a low-ranking member) outweigh the costs (Cheney and Seyfarth 1987).
Predator Avoidance
While D. L. Cheney and R. M. Seyfarth (1987) found that larger vervet groups had higher average infant and female survival rates, causes of mortality differed based on group size. Unlike the small group, mortality in larger groups was almost entirely due to predation, and this highlights another set of costs and benefits of group living. Larger groups are more conspicuous than smaller groups. This is one of the reasons that primates who rely on crypsis to avoid predation (like the slow loris; Figure 6.16) are often solitary. However, some anti-predator behaviors, like shared vigilance duties, alarm calling, and mobbing, are responses to predators that are only available to group-living species (like Hanuman langurs; Figure 6.17). Whether or not a primate is group-living or solitary, it engages in some form of , or watchful behavior to detect potential danger. Often, researchers cannot determine whether vigilance is intended to detect predators or potential competing conspecifics (with predator detection as a side benefit). However, because vigilance interferes with other important behaviors like feeding, resting, or being social, primates who live in groups benefit from sharing the cost of vigilance and reaping the rewards of early predator detection. When a predator is detected, an alarm call is given. We will discuss the information communicated through alarm calls in greater detail in the “Communication” section, but in short, they serve one of two functions: (1) to alert members of the group to the presence of a predator or (2) to alert the predator that it has been detected. In some species, (the act of cooperatively attacking or harassing a predator) accompanies alarm calls. Mobbing involves two or more individuals making repeated advances on a predator, often while vocalizing and/or displaying. The point of mobbing is to drive off or distract the predator long enough for others to escape. Primates have been observed mobbing several species of predators, including chimpanzees, leopards, and eagles, but snakes are the most common targets. Although mobbing often occurs as the predator is approaching, in some cases, it occurs after a predator has attacked and escalates to a counter-attack. A group of Coquerel’s sifaka mobbed a Madagascar ground boa that had grabbed and was constricting an adult female. The attack, which consisted of loud alarm calls, along with multiple individuals biting and scratching the snake’s body and head, resulted in the snake releasing the female sifaka, who survived (Gardner et al. 2015). Similar reports of mobbing resulting in the rescue of a group member from the coils of a boa constrictor have also been reported for white-faced capuchins and moustached tamarins. Such examples clearly illustrate the benefits of group living.
Polyspecific Associations
In regions with a large number of sympatric primate species (Figure 6.13), interactions between species are bound to occur. Often interactions are competitive (more on this in the “Competition for Food” section). However, are different. These are associations between two or more different species in which at least one species changes its behavior to maintain the association. Polyspecific associations have been documented in many New World and Old World primate communities. While some associations are short in duration, others can be semi-permanent. In these cases, species are found more often in association than not. As discussed above, decades of research indicates that primates obtain benefits from living in groups with conspecifics. So why do some primates form associations with other species instead of increasing the size of their own group? Although the specific costs and benefits of polyspecific associations differ in each case, in general, species that form these associations gain foraging or anti-predator benefits while avoiding within-group competition for food that occurs in a larger group of conspecifics.
There are many possible foraging benefits of polyspecific associations. In some cases, one species gains access to a food resource that is otherwise inaccessible. In Manu National Park, in Peru, brown capuchins chase smaller squirrel monkeys away from scarce resources. Despite this, squirrel monkeys maintain the association because the capuchins can crack open palm nuts that squirrel monkeys cannot. Squirrel monkeys then feed on kernels dropped by the capuchins (Terborgh 1984). In Brazil, saddle-back tamarins obtain a slightly different foraging benefit by associating with moustached tamarins. The larger (in body and group size) moustached tamarins flush insects from the upper canopy as they forage. The fleeing insects are captured at high rates by saddle-back tamarins foraging below them (Peres 1992). In other cases, associated species avoid competition for food. In Makokou, Gabon, associations form between greater spot-nosed guenons, moustached guenons, and crowned guenons, despite the fact that these closely related species have very similar diets. Instead of competing for food, the species benefit from reduced indirect competition. Because they encounter food sites together, they avoid visiting a site that might have been depleted by one of the other species if they were foraging separately (Gautier-Hion et al. 1983).
In other cases, the benefit of polyspecific associations is predator avoidance. Like foraging benefits discussed above, anti-predator benefits are variable. In some cases, one species may be particularly good at detecting a specific type of predator and may alert the other species to its presence. In Makokou, Gabon, the guenon species discussed above play different alarm call roles when associated (Gautier-Hion et al. 1983). Moustached guenons, who spend more time close to the ground, are usually the first to alarm call at terrestrial predators. Crowned guenons, who spend more time high in the forest canopy, are most likely to detect aerial predators. Because both species give an alarm call familiar to the other species in the association, everyone benefits from increased predator detection. Sometimes associations result in proactive defense against predators. In the Una Biological Reserve in Bahia, Brazil, a mixed-species group of golden-headed lion tamarins and black-tufted ear marmosets was observed jointly mobbing an ocelot (Raboy et al. 2008). In Taï National Park in Côte d’Ivoire, putty-nosed guenons join Diana monkeys in coordinated mobbing of crowned eagles (Eckardt and Zuberbühler 2004).
Dispersal: Who Goes, Who Stays, and Why?
Whether primates live in groups or are solitary, some individuals must disperse, or leave the place or group of their birth. In the solitary orangutan, females spend about seven years caring for each highly dependent offspring. But once mature, offspring of both sexes leave their mother’s home range. If this did not happen, orangutans would not be solitary. In group-living species, one or both sexes must disperse at sexual maturity. Which sex disperses depends on the relative costs and benefits to each. In most primate species, males are the dispersing sex because the benefits of dispersal, including increased access to mates and reduced competition from other males, outweigh the costs. For most female primates, the opposite is true: they usually benefit from remaining , or in the group of their birth. This allows them to maintain strong social alliances so that they can compete successfully against other groups for food. In species where females are typically philopatric, like vervets and macaques, female dispersal only occurs under extreme circumstances, such as when group size falls to precariously low levels. Despite the patterns discussed below, it is important to remember that there is considerable variation in dispersal and numerous exceptions to any rule. Although uncommon, female dispersal has been observed in typically female philopatric species like capuchins and baboons. Likewise, female philopatry has been recorded in species like chimpanzees and muriquis, whose females typically disperse. These exceptions underscore the high degree of behavioral variation and flexibility displayed by primates.
Costs of Dispersal
Transferring into a new group can be fraught with difficulties. Members of both sexes may experience aggression from same-sex members of their chosen group because they are viewed as potential competitors. Aggression toward transferring individuals has been documented in multiple species, and aggression directed toward transferring males is almost universal and can be lethal (Isbell and Van Vuren 1996). During my fieldwork in Kenya, a subadult male patas monkey who had recently dispersed attempted to return to the group into which he was born, which happened to be our study group. The resident male attacked him and severely wounded him. We did not see the subadult male again and assume he died. Transferring females can also experience aggression. Female red howler monkeys are often prevented from joining established groups and can be injured by resident females when they attempt to do so (Crockett and Pope 1988). Even if new group mates are not aggressive, the dispersing individual has lost all alliances with members of their old group and must expend time and energy developing relationships with members of the new group. New group members are often lower in the dominance hierarchy and may produce fewer offspring and suffer from greater mortality. Individuals who disperse into an unfamiliar home range must contend with a lack of ecological knowledge. For species who feed on clumped and seasonal resources like fruit, the lack of knowledge about food sites in a new area can be a significant cost. Lack of knowledge about predators can also put dispersing individuals at greater risk, as appears to be the case for vervets. When their trees deteriorated, vervets in Amboseli National Park, in Tanzania, began to shift home ranges. Use of unfamiliar areas correlated with an increase in vervet disappearances. Most were suspected to have died from leopard predation, probably due to a lack of knowledge about escape routes and refuges in unfamiliar areas (Isbell et al. 1990). Individuals who lose both social allies and knowledge of a specific area when they disperse may suffer even higher costs (Isbell and Van Vuren 1996).
Benefits of Dispersal
If the costs are so high, why do individuals disperse at all? The answer to this question depends on whether we look at the immediate cause of dispersal or the reproductive consequences over the long term. In the short term, the cause of dispersal is often eviction by same sex members of the group, as occurs in gibbons, ring-tailed lemurs, red howler monkeys, and other species. In Hanuman langurs, the resident male may be kicked out by bachelor males who invade heterosexual groups during the breeding season. In other cases, maturing individuals may choose to leave their group because they are attracted to individuals in another group. This explanation is supported by the observation that most transfers by males between groups occur during the breeding season, when females are sexually , or ready to mate. Among hamadryas baboons of Ethiopia, one cause of female dispersal is abduction of juvenile females by adult males. The male incorporates the female into his harem and mates with her when she reaches adulthood (Swedell and Schreier 2006). In chimpanzees, females disperse because males gain significant benefits from remaining in their (the group into which they are born). These benefits include hunting cooperatively and patrolling the community boundary together (Lutz et al. 2016; Stumpf et al. 2009). Other explanations for dispersal are related to enhancing , or one’s genetic contribution to future generations, often measured through number of offspring produced. A male may disperse to enter a group with fewer same-sex individuals, so as to avoid competition for mates. Likewise, dispersing into a group with more members of the opposite sex can increase an individual’s mating opportunities. Perhaps the most common explanation for dispersal of at least one sex from the perspective of reproductive success is to avoid , or mating with close relatives. When close relatives mate, the likelihood that the offspring will inherit two copies of a recessive gene increases. If the trait that these recessive genes code for is harmful, then such matings can result in , or reduced fitness of the population. Evidence for inbreeding avoidance as an explanation for dispersal includes the fact that , or dispersal out of the group of one’s birth, takes place at sexual maturity and that at least one sex always disperses.
REPRODUCTIVE STRATEGIES
It is important to recognize that primate reproductive strategies have evolved to maximize individual reproductive success. These strategies are divided into those dealing with offspring production and care (parental investment) and those that maximize mating success (sexual selection). Because the reproductive physiology of male and female primates differs (males produce sperm and cannot gestate or lactate; females produce eggs and gestate and lactate), males and females differ with regard to parental investment and sexual selection strategies. Female strategies, on the one hand, focus on obtaining the food necessary to sustain a pregnancy and choosing the best male(s) to father offspring. Male strategies, on the other hand, focus on obtaining access to receptive females.
Parental Investment
Biologically speaking, is any time or energy a parent devotes to the current offspring that enhances its survival (and eventual reproductive success) at the expense of the parent’s ability to invest in the next offspring (Trivers 1972). Female primates invest more heavily in offspring than males. Even before conception, females produce energy-laden eggs, and will be responsible for sustaining a fertilized egg until it implants in the uterus. After that, they invest in pregnancy and lactation (Figure 6.1a). Because all of this investment is energetically expensive, female primates can only produce one offspring (or litter) at a time. A species’ interbirth interval is determined by the length of time necessary to maximize each offspring’s survival without jeopardizing the female’s ability to produce the greatest number of offspring possible. If a female invests too little (i.e., weans an offspring too early), she may give birth to many offspring, but very few (if any) of them will survive. If she invests too much (i.e., nurses an offspring even though it could be weaned), she ensures the survival of that individual offspring but will not be able to produce very many during her lifetime. To maximize her reproductive success, a female must invest just long enough to ensure the greatest number of offspring survive to reproduce.
We often think of maternal care as a natural, instinctive behavior. Yet this is not the case. Zoos, for example, almost always have nurseries where infants are cared for by zookeepers if their mothers will not care for them. These exhibits are among the most popular because the babies are so cute and so much fun to watch. And the caretaking positions in zoo nurseries are often among the most coveted by zoo personnel for the same reasons. But if maternal behavior is instinctive, why do zoo nurseries even exist? The answer is that in many species, including primates, maternal behavior is not purely instinctual; it is dependent on (behavior learned by observing and imitating others), as well. Captive female primates, including gorillas and chimpanzees, who have not had the opportunity to observe their mother or other females care for infants do not know how to care for their own offspring. Although it is preferred that the mother care for her infant, in cases when she will not, humans must step in to ensure the offspring survives. When hand-rearing by humans is necessary, the infant is returned to the group as soon as possible in the hopes that it will learn species-typical behavior from its mother and other conspecifics. Observations such as these indicate that maternal behavior is learned, not innate, and that maternal care is critically important to the social and psychological development of young primates.
Although females invest more in offspring than males, there are some conditions under which males will invest. Male investment takes many forms, ranging from carrying or grooming infants to sharing food with them, to protecting them from (killing of infants of one’s own species) or predators, to simply tolerating their presence. A male who has some degree of , or confidence that he is the father, is more likely to invest in an offspring than a male who does not because any investment in the offspring may increase his own reproductive success. Males appear to use a very simple rule, “Have I recently mated with this infant’s mother?”, to determine their paternity certainty. For example, male mantled howler monkeys only care for infants they may have fathered while Hanuman langur males protect and never attack infants who might be their own (Borries et al. 1999; Clarke et al. 1998). During my fieldwork in Kenya, I observed the first suspected case of infanticide in patas monkeys (Enstam et al. 2002), committed by the only resident male over a 10-year study period who took over the group too late in the breeding season to have fathered any of the offspring. It is certainly not a perfect rule, and males may sometimes invest in an offspring they did not father. However, this is less costly than killing your own infant.
Sexual Selection
, or selection for traits that maximize mating success, comes in two forms. is selection for traits that enhance the ability of members of one sex to compete amongst themselves (“intrasexual” = within one sex). is selection for traits that enhance the ability of one sex to attract the other (“intersexual” = between the sexes). Intrasexual selection most often operates on males. In the wild, adult females are either pregnant or lactating for most of their adult lives. So the ratio of sexually available males to sexually receptive females (the ) usually includes more males than females. The result? Receptive females are a scarce resource over which males compete. Intrasexual selection favors traits that make a male a better competitor (i.e., a winner). Competition between males (hereafter referred to as male-male competition) can take many forms but comes in two main categories: direct competition and indirect competition (just like competition between females for food). Intersexual selection also tends to operate on males, selecting traits that make a male more attractive to females. Females, in turn, choose among potential fathers. Because female primates invest more in offspring production and care than males (see the “Parental Investment” section), they pay a significantly higher cost if the offspring dies before maturity or reaches maturity but does not reproduce. Thus, it benefits a female primate to be choosy, and this requires males to display traits that tell a female why she should choose him, and not another male, as her mate.
Intrasexual Selection: Competition for Mates


If females live together in groups, a male (or males) may be able to monopolize access to them. Under such circumstances, intrasexual selection favors traits like large body size (Figure 6.19a) and large canines, which increase a male’s competitive ability in fights with other males. Because females don’t possess these same traits, males and females of some species look different, which is called (Figure 6.19a). We will discuss sexual dimorphism in greater detail in the next section. In some species, a single, highly competitive male is able to defend a group of females from other males. Males may use vocalizations, displays, or physical combat to defend their group of females from extra-group males. In other species, it is impossible for a single male to monopolize a group of females. In these species, groups contain multiple females and multiple males. In combat between two males, the stronger, larger male is more likely to win, all else being equal. However, when groups contain multiple males, males have the opportunity to form , or temporary alliances to cooperate in an effort to enhance their competitive ability.


If one male cannot keep another from mating with a female, indirect competition occurs. Indirect competition can take many forms, but in all cases, the males do not interact; they may, in fact, never even see each other. occurs when multiple males mate with the same female in relatively close succession. Under such circumstances, the male that produces the greatest quantity of long-lived sperm should have a better chance of fertilizing the female’s egg. Evidence for sperm competition comes from correlations between mating system and testes weight, which is used as a proxy for sperm production (Harcourt et al. 1981). Take chimpanzees and gorillas as an example. Chimpanzees live in groups with multiple adult males and females while gorilla troops contain one adult male (the silverback) and multiple females (for more information on social and mating systems, see the next section). Because male chimpanzees cannot keep others from mating with females, producing greater quantities of sperm is perhaps their best way to ensure paternity. Male gorillas who are able to monopolize a group of females (through direct competition with other males) do not need to compete with sperm, and so they do not need to produce it in large amounts. Therefore, although male gorillas are much larger in body size, male chimpanzees have larger testicles to produce more sperm. In other species, males engage in alternative mating strategies. Orangutans are socially solitary, but a single large adult male’s territory overlaps the territories of multiple females. The male actively keeps other males out and away from the females. A non-territorial male may compete directly with a territorial male, but this is dangerous and can result in serious injury. Some males avoid this by delaying the development of , or traits associated with sexual maturity. In orangutans, these traits include large cheek phalanges, a throat sac, and large body size (Figure 6.20a). Males that do not develop these traits look like juveniles (Figure 6.20b) and seem to use their non-threatening appearance to sneak into the territories of fully developed males to mate with females. The mechanism that results in the two male morphologies is not well understood, but males lacking secondary sexual characteristics have lower testosterone levels (Marty et al. 2015). Lastly, males may compete indirectly by committing infanticide. Infanticide occurs in many primate species, including red howler monkeys, chacma baboons, crab-eating macaques, diademed sifakas, ring-tailed lemurs, Hanuman langurs, and mountain gorillas. If a male kills a competitor’s infant, the mother will resume ovulation more quickly, providing the infanticidal male with an opportunity to father her next infant. Thus, under the right circumstances, an infanticidal male benefits by removing his competitor’s genes from the gene pool while adding his own to it.


Although more rare than male-male competition, sometimes females compete for mates. The callitrichids, the primate family that includes marmosets and tamarins, are unusual in their reproductive pattern. Breeding females often give birth to twins (Figure 6.21a–b), sometimes producing litters twice a year. Another interesting characteristic of callitrichid reproductive behavior is the fact that often only one female reproduces, a phenomenon that is achieved through (Digby et al. 2011). The mechanisms differ across species but generally involve the prevention of reproduction by subordinate females through physiological and/or behavioral means. These subordinate females are often the older daughters of the breeding female. In some species, the dominant female emits chemicals that delay ovulation in subordinates. In others, she physically breaks up matings between males and subordinate females. Regardless of the exact mechanism, the goal is the same: to limit the opportunities for subordinate females to become pregnant. But why? Although a breeding female can give birth to triplets or quadruplets, it is rare for more than two offspring from each litter to survive. Even ensuring the survival of twins is more than the mother can manage by herself. To maximize her offsprings’ survival, she needs all group members, including other (reproductive-age) females, to care for her offspring instead of focusing on their own. It is clear that this strategy helps the breeding female’s reproductive success. But why would her reproductive-age daughters “agree” to stay in their natal group and help mom raise their siblings instead of dispersing to another group and breeding themselves? If a subordinate female cannot find a group to transfer into as the breeding female, she has two options: stay in her natal group and raise younger siblings, or transfer to another group as a subordinate and raise the offspring of a female to whom she is not related. Because she shares genes with her siblings (50% if they are full siblings, 25% if they are half siblings), some of the subordinate female’s genes get passed down if her siblings survive and reproduce. On the one hand, fewer of the female’s genes get passed down through siblings (called ) than if she had produced her own offspring (called ). But, on the other hand, she passes on more genes by raising her siblings than if she helped to raise the offspring of a female to whom she was not related. Not surprisingly, subordinate females rarely leave their natal group unless a breeding position opens in another group.
Intersexual Selection: Mate Choice
As we discussed at the beginning of this section, female primates are choosy because it is more costly for them (in terms of reproductive success) if they produce an offspring that either does not survive or that survives but cannot or does not reproduce. But what is it that they are choosing in males? Like all other examples of primate behavior and ecology, there is both species-level and individual-level variation in female choice. In many animals, including humans, females choose a male who can provide important resources, such as food, paternal care, or protection. Examples of such direct benefits are rare in primates, since most females do not require males to supply them these resources. Female mountain gorillas and chacma baboons, however, may choose males based on who can protect them from infanticidal males (Henzi and Barrett 2003; van Schaik and Kappeler 1997). More commonly, female primates obtain indirect (i.e., genetic) benefits from choosing one male over another. Often the specific criteria by which females select mates is unknown. However, if a female chooses a healthy (as indicated by traits like a plush coat, bright coloration, or large body size) or older male, she may obtain genes for her offspring that code for health or longevity. If a male’s rank is determined by competitive ability that has a genetic component, females who choose such males may acquire these genes (and qualities) for their offspring. Females in some species appear to prefer new immigrants, sometimes even “sneaking” copulations with males who are not established members of their groups. Such a preference may provide their offspring with novel genes and increase genetic variation (for more about the importance of genetic variation, see Chapter 4). Lastly, female choice does not necessarily imply that females are choosing only one male with whom to mate. In many species, females actively choose to mate with multiple males. The most likely explanation for this phenomenon is an attempt to avoid infanticide by ensuring that multiple males think they are possibly the father. This is called . In such cases, females may not be choosing mates based on direct (resource-based) or indirect (genetic) benefits but, rather, ensuring that any male who might be in close proximity to her infant after birth will not kill it.
Female choice is often more subtle than male-male competition, so it can be more difficult to study. However, as more research is conducted, we better understand the ways that female primates exert their choice. In many species, females actively solicit sexual interactions with some males and not others. In other cases, females reject advances by some males and not others. Grey-cheeked mangabeys in Kibale National Park, Uganda, exert female choice in multiple ways (Arlet et al. 2007; Smith 1994). They present their hindquarters (which signals interest in mating) significantly more to high-ranking and immigrant males; they refuse to mate with some males; and most mate with multiple males when they are receptive. These results indicate that rather than being passive actors who accept matings with eager males, female primates actively participate in choosing amongst suitors.
Social and Mating Systems
Sometimes the terms social system and mating system are used interchangeably, but there are important differences between the two terms. A describes the typical number of males and females of all age classes that live together. A describes which male(s) and female(s) mate. Two species can have the same social system but a different mating system and vice versa. For example, the mating system of both orangutans and mountain gorillas is —that is, one male mating with multiple females—but the social systems of these two great apes is very different. The home range of one large adult male orangutan overlaps the home ranges of many females, with whom he mates, but they do not travel together as a cohesive group (Figure 6.22a). Mountain gorillas travel in cohesive one-male, multi-female groups consisting of a silverback male, multiple females, and their dependent young, and the silverback male mates with the females of his group (Figure 6.22b). So how is it that two species can have the same mating system and different social systems or, alternately, the same social system and different mating systems? It all depends on how food, females, and males are distributed.
We can understand primate social and mating systems by thinking of layers of a map. The first layer is food distribution. Because female reproductive success is limited by access to food, females “map onto food” and form the second layer of the map. If food exists in large clumps that can feed multiple individuals (like fruit), females can also exist in “clumps” (i.e., groups) and will benefit from doing so because living in groups helps with defense of food sources. Finally, because male reproductive success is limited by access to females, males “map onto females” forming the third layer of the map. If females live in cohesive groups, one or a few males have the opportunity to monopolize them. If females are widely distributed, it is more difficult (sometimes impossible) for males to monopolize multiple females.
Key: ![]() = adult male;
= adult male; ![]() = adult female; open black circle represents the outline of the male’s home range (solitary species) or group’s home range; open red circle represents individual female home ranges (solitary species). Illustrations by Karin Enstam Jaffe.
= adult female; open black circle represents the outline of the male’s home range (solitary species) or group’s home range; open red circle represents individual female home ranges (solitary species). Illustrations by Karin Enstam Jaffe.
 |
 |
 |
|
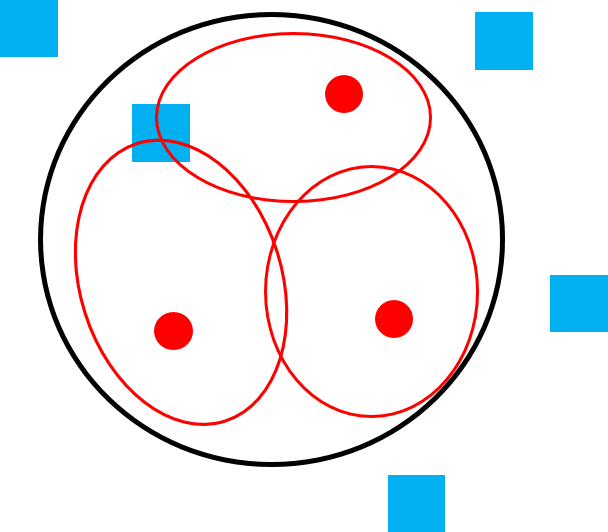
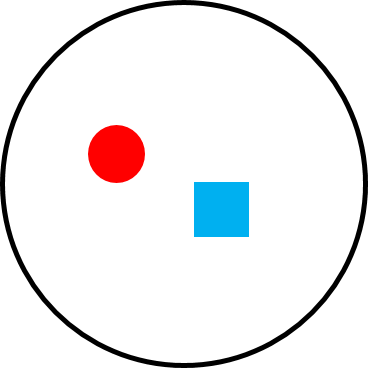
Figure 6.22a Polygyny in a solitary species, like orangutans. |
Figure 6.22b Polygyny in a group-living species with a single-male, multi-female social group, like mountain gorillas. |
Figure 6.22c Monogamy in species that form family groups, like gibbons. |
 |
 |
|
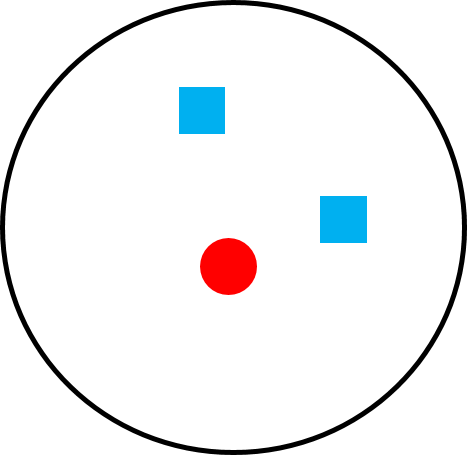
Figure 6.22d Polyandry in species like tamarins and marmosets. |
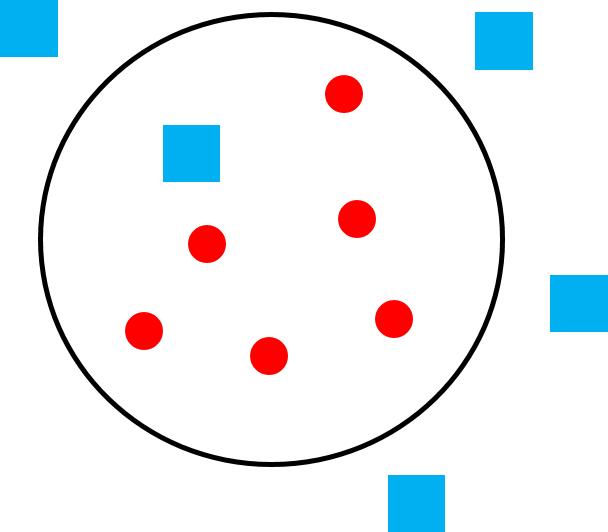
Figure 6.22e Polygamy in species that live in multi-male, multi-female groups, like vervets. |
When Females Are Solitary
When food is distributed in such a way that females are unable to live in close proximity to each other, they must spread out to avoid too much competition. A male may choose to guard one female, try to monopolize multiple females by himself, cooperate with other males to monopolize multiple females, or cooperate with other males to help raise the offspring of an individual female. The difference in these male strategies is illustrated by the gibbon, orangutan, chimpanzee, and tamarin.
Both gibbon and orangutan females eat fruit found in relatively small patches that does not support groups, so females of both species are solitary. However, the way in which males map onto the distribution of females is quite different. A male gibbon guards a single female, resulting in a mating system (Figure 6.22c). A pair of gibbons form a long-term bond that includes defending a territory and relatively high paternity certainty that results in male care of offspring. Mated pairs defend their territory by calling together in a patterned vocalization called a duet. These coordinated vocalizations tell other gibbons that the territory is occupied and to stay away. Because most males get a mate, male-male competition is relaxed, and there is little pressure for males to develop large body size or weaponry to use in competition with other males. Thus, it is not surprising that male and female gibbons exhibit , meaning that males and females are similar in body size and often look alike. Because males and females both exclude same-sex competitors, the social group consists of an adult male, an adult female, and their dependent offspring, sometimes referred to as a family group.
Like gibbons, orangutan females are also solitary. But unlike gibbon males, who cannot monopolize access to multiple females, a male orangutan has a very large home range that overlaps the home ranges of two or more females (Figure 6.22a). The females do not regularly travel with each other or the male, but he mates with them, resulting in a polygynous mating system but a solitary social system. Because some males monopolize multiple females, many male orangutans do not have access to females. Male-male competition is intense, and males benefit from large body size, weaponry, and other traits that increase their competitiveness. The result is significant sexual dimorphism. Male orangutans are twice the size of females and have large canines, cheek phalanges, and throat sacs (Figure 6.20a) that help them defend their home range (and females) through direct (fighting) and indirect (territorial vocalizations) competition with other males. As we discussed in the previous section, the competition is so intense that some males remain in a state of arrested development (Figure 6.20b).
Chimpanzees have a fluid social system referred to as fission-fusion. When food is plentiful, female chimpanzees of the same community travel together within their community territory. When food is scarce, the group “fissions” and females travel independently, with their dependent offspring, in their own range but still within the community territory. Because male chimpanzees are philopatric and related to one another, they share more genes in common than males in other primate species who are unrelated. The high degree of relatedness results in high levels of cooperation (see the discussion of chimpanzee cooperative hunting in the “Why Do Primates Live in Groups?” section) and reduced sexual competition between males. Even males who do not father their own offspring have some genes passed on by male relatives who do (this is another example of indirect fitness). Male chimpanzees do compete to be at the top of the dominance hierarchy so as to obtain priority of access to females. However, no male in the community is excluded from mating with community females, so chimpanzees practice as a mating system (in which multiple males mate with multiple females), even though females are solitary for some of the year. Competition between males is relaxed because they are related and all get to mate. This results in reduced sexual dimorphism. Unlike orangutan males, male chimpanzees are only about 25% heavier than females. But like orangutans, male chimpanzees compete indirectly, particularly through sperm competition.
Although there are many examples of multiple males living in groups with multiple females (we’ll discuss some examples below), it is rare for multiple males to live with a single breeding female, a mating system referred to as (Figure 6.22d). Yet this is the pattern we often see in the callitrichids: tamarins and marmosets. As we discussed in the “Parental Investment” section, due to their rapid reproductive rate and propensity for twinning (Figure 6.21a–b), breeding females need help from all group members to raise their offspring, and they suppress reproduction in other females in their group, effectively making breeding females solitary. In some callitrichid species, the dominant male fathers most or all of the offspring, but the males in the group are relatives so they benefit genetically, similar to chimpanzee males (Baker et al. 1993). In other species, males are not related, but the breeding female mates with all the males in the group, so every male has a chance of being the father of the offspring (Díaz-Muñoz 2011). In both cases, males help rear offspring because they cannot afford not to do so. Although social systems differ across tamarin and marmoset species, and even across populations of the same species, polyandry is common among callitrichids but extremely rare in other primates.
SPECIAL TOPIC: WOMEN IN PRIMATOLOGY: MEET “THE TRIMATES”
While many STEM (Science, Technology, Engineering, and Math) fields have traditionally been, and continue to be, dominated by men, primatology has a long history of significant research conducted by women. This is due, in part, to the fact that three of the most well-known primatologists are women, making it clear that this is a field in which women can excel. In the early 1960s, British paleoanthropologist Louis Leakey (see Chapters 9 and 10 for more about Louis Leakey’s work) was looking for students to study the great apes in hopes of shedding light on the behavior of our early ancestors. He chose Jane Goodall (Figure 6.23a) to study chimpanzees, Dian Fossey (Figure 6.23b) to study mountain gorillas, and Birute Galdikas (Figure 6.23c) to study orangutans. These three women, sometimes referred to as Leakey’s “Trimates,” have transformed our understanding of ape (and primate) behavior through their work.

Arriving at the Gombe Stream Reserve in Tanzania in 1960, Jane Goodall (Figure 6.23a) was the first scientist to conduct a long-term study of wild nonhuman primates. Until then, most field studies lasted less than a year. By 1961, she had made two astounding observations that forced us to reconsider what differentiates humans from the rest of the primate order: She observed chimpanzees eating a colobus monkey, the first reported evidence of meat eating in our closest relatives (she later observed them hunting and killing other mammals and sharing the meat) and also discovered that chimpanzees make and use tools by stripping leaves off twigs to “fish” for termites. After several decades of study, her work has produced long-term data on chimpanzee mating strategies, mother-infant bonds, and aggression within and between communities. When her study group, the Kasakela community, fissioned in the mid-1970s, she observed males of the larger community attack and kill those of the smaller one. This behavior, which Goodall compared to human warfare, is now known to be typical of wild chimpanzees and is another behavior we share with our closest relatives. In the mid-1980s, Goodall transitioned from field researcher to conservationist and activist, advocating for the humane use of nonhuman animals (Stanford 2017).

In 1967, Dian Fossey (Figure 6.23b) began her long-term study of mountain gorillas and founded the Karisoke Research Center in Rwanda. Through patience and hard work, Fossey habituated several groups of gorillas to the presence of human observers, and their research over several decades has formed the foundation of our understanding of gorilla social behavior, ecology, and life history. Gaining the gorillas’ trust was difficult as they were fearful of humans they had known only as poachers. Censuses of the Virunga gorilla population in the 1970s by Fossey and her colleagues estimated a population of fewer than 300. This represented a decline of 40% from the previous decade. The primary causes of this decline were habitat loss and illegal hunting. Fossey’s advocacy for mountain gorilla conservation kicked into high gear when, at the end of 1977, poachers killed her favorite gorilla, Digit, as he protected his group. A year later, poachers attacked one of her main study groups and killed several gorillas as they tried to kidnap an infant to sell to a zoo. Her efforts to publicize the killings led to the development of conservation programs that ultimately saved the mountain gorilla. By the end of the 1980s, the population had begun to recover and continues to increase. Tragically, Dian Fossey was murdered in her research cabin at Karisoke in December 1985; the case remains unsolved (Stewart 2017).

Birute Galdikas (Figure 6.23c) began her study of orangutans in Kalimantan, Borneo, in 1971 and set up a field station called Camp Leakey. Hers was the first long-term study conducted on the Bornean orangutan. Her research still continues, and over 150,000 hours of observational data have been collected by Galdikas and her colleagues, focusing on the life histories of individual orangutans. While conducting her behavioral research, Galdikas discovered that the pet trade and habitat loss were adversely affecting the orangutan population. She began working with the Indonesian government to confiscate orangutans that had been removed from the wild illegally, many of whom ended up as pets. Taking these orphaned orangutans back to Camp Leakey, Galdikas’s conservation efforts began to extend beyond advocacy and into rehabilitation and forest preservation (Bell 2017). If you would like to learn more about primate conservation, please see Appendix B.
When Females Live in Groups
When females live together, either because their food is abundant (in the case of folivores) or because their food is distributed in large patches that are worth defending (in the case of frugivores), males have the opportunity to monopolize multiple females. Sometimes a single male is able to monopolize a group of females. Other times, a male may not be able to exclude other males from the group.
Generally speaking, when female groups are small and cohesive, it tends to be easier for a single male to monopolize a group of females. Mountain gorillas, hanuman langurs, red howler monkeys, and patas monkeys are examples of single-male, multi-female groups, which consist of one adult resident male, multiple adult females, and their dependent offspring. The mating system for single-male, multi-female groups is polygyny (Figure 6.22b). Because a relatively small number of males monopolize all the breeding females, there are many adult males who do not have mates. As with orangutans, this results in strong competition between males, resulting in sexual dimorphism where males are much larger than females. In mountain gorillas, fights between silverbacks can be intense. Males can use their large canines to cause serious wounds that may even result in death (Fossey 1983).
When a single male cannot monopolize a group of females, often because the group consists of many females that may be spread out over a wide area, the result is a multi-male, multi-female group consisting of multiple adult males, multiple adult females, and their dependent offspring (Figure 6.22e). Olive baboons, ring-tailed lemurs, and squirrel monkeys are examples of primate species with this type of social system. Because a single male cannot exclude others in the group from mating, the mating system in multi-male, multi-female groups is polygamy, but that does not mean that all males have equal reproductive success. When multiple males live in a group, they often form a dominance hierarchy that determines their priority of access to females in the group. This is similar to the way a female dominance hierarchy determines a female’s priority of access to food. Because their place in the hierarchy can affect their reproductive success, males in multi-male groups engage in male-male competition, but because it is rare for males to be excluded from mating altogether, the level of competition, and degree of sexual dimorphism, is less extreme than what we see in polygynous species.
COMMUNICATION
In its most basic form, communication occurs when one individual (the sender) emits a signal that conveys information, which is detected by another individual. We have discussed several aspects of primate sociality in this chapter, all of which require the communication of information between individuals. But, how does a female chimpanzee communicate her sexual availability? How does a vervet monkey communicate the approach of a leopard or that a python is nearby? How does a dominant (or subordinate) macaque signal its place in the dominance hierarchy? How do solitary, nocturnal primates, like the slow loris, communicate information about themselves to conspecifics?
Forms of Communication
Primate communication comes in four forms: vocal, visual, olfactory, and tactile. Species vary in their reliance on each. Because it is difficult to see others in the dark, and because nocturnal primates avoid predators by remaining quiet, species like the slow loris and the aye-aye rely heavily on scent-marking to communicate with conspecifics. Diurnal species tend to rely more heavily on visual and vocal forms of communication.
Vocal Communication
Primates use sound to claim and maintain a territory, make contact with other group members, or to communicate danger or threats, among other things. Loud calls are designed to travel great distances and are used in territorial defense by many primate species including indris, orangutans, gibbons, howler monkeys, and siamangs. In dense forest, where visual communication can be difficult, loud calls can be useful in signaling to conspecifics that a group or individual occupies a specific area. Howler monkeys are named for their loud calls, or “roars,” which can be heard one kilometer or more away (Schön Ybarra 1986). Howler group roars may act to maintain distance between neighboring groups or keep extra-group males from entering the home range (Schön Ybarra 1986; Sekulic 1982).
Other vocalizations are intended to communicate with individuals in one’s own group. These include vocalizations given as part of threat displays or dominance interactions, as well as contact calls that provide information about location to other group members. Baboons have a rich repertoire of vocalizations for communicating with other group members (Fischer et al. 2008; Ransom 1981). Adult males give specific vocalizations during threat displays and physical confrontations. Subordinates “screech” when retreating from a dominant individual, signaling submission. Since baboons rely on membership in their group for finding food and detecting predators, a baboon separated from his group will vocalize in an attempt to regain contact. Young baboons emit their own contact calls when separated from their mothers.
Alarm-calling behavior is widespread in primates. Often, alarm calls serve to notify conspecifics of potential danger, as is the case with vervet monkeys (Figures 6.4a, 6.11b). Research by Dorothy Cheney, Robert Seyfarth, and others has shown that: (1) vervets classify predators based on hunting style; (2) alarm calls convey information to other vervets about that hunting style; and (3) other vervets respond in ways appropriate for evading that type of predator (Seyfarth et al. 1980a). When a vervet gives a “leopard” alarm call [directed at mammalian carnivores like leopards (Figure 6.15a) and dogs], monkeys on the ground climb the nearest tree, while monkeys already in trees stay there or climb higher. Since most mammalian carnivores hunt on the ground, getting into, and staying in, a tall tree is the best option for escape. When the distinct “snake” alarm call is given, vervets stand on their hind legs and look down at the ground. Since snakes, like pythons (Figure 6.15g) are not pursuit predators, locating them so as to avoid them is the best strategy. Lastly, when an “eagle” alarm call is given, vervets look up or run into bushes, both of which are useful responses for avoiding hawks and eagles (Figure 6.15e), which attack from above. Vervets clearly understand the meaning of each type of alarm call, as they respond appropriately even when they do not see the actual predator (Seyfarth et al.1980b). Such , which involves the systematic use of signals to refer to objects in the environment, was once believed to be unique to humans. It may be a precursor to the symbolic capacities of human language.
Research on other African monkeys indicates that some species use alarm calls to signal to the predator that it has been detected. Diana monkeys, Campbell’s guenons, and sooty mangabeys of the Taï forest give alarm calls to leopards but not chimpanzees (Zuberbühler et al. 1997). Because leopards (Figure 6.15a) are stealth predators, they rely on the element of surprise to sneak up on their prey. Alarm calling at leopards appears to tell the leopard that it has been seen and therefore its chance of success will be low. Leopards are more likely to stop hunting after an alarm call has been emitted. Unlike leopards, chimpanzees are pursuit predators and may even use alarm calls to locate potential prey. With such a predator, prey are better off remaining as silent as possible so as not to alert the predator to their location (Boesch and Boesch 1989; Zuberbühler et al. 1999).
Visual Communication
Visual signals are an important component of nonhuman primate behavior, alone or in combination with other forms of communication. Some visual signals are common to all nonhuman primates. For example, , or raising one’s hair or fur, is used in aggressive interactions to make an individual appear larger than it actually is. The females of many Old World primate species, including macaques, baboons, and chimpanzees, signal sexual receptivity through changes in the size, shape, and, often, color of their hindquarters, called a (Figure 6.24a). The sexual swelling reaches its maximum size at ovulation. When females are not receptive, either because they are pregnant or are nursing, they do not display a sexual swelling (Figure 6.24b). Thus, its presence or absence signals a female’s reproductive state. In some species, females use other visual cues to indicate sexual receptivity. Common marmoset females solicit mating through tongue-flicking displays directed at males, while female patas monkeys engage in a more elaborate visual display. When soliciting mating, the female crouches in front of the male and looks back at him while blowing air into her cheeks; she also may drool and curl her tail (Chism et al. 1984).




Monkeys and apes also use a diversity of facial expressions in visual communication. Showing your teeth in a “smile” sends a signal of friendship in humans. Displaying teeth in this way is a sign of anxiety or fear in Old World monkeys. That male mandrill you see “yawning” at your local zoo is actually displaying his teeth to signal tension or threaten a rival (Figure 6.25). Male gelada baboons use “lip flips,” in which the gums and teeth are exposed by flipping the upper lip up over the nostrils (Figure 6.26), and “raised eyelids,” in which the pale eyelids are exposed by pulling the scalp back as threatening gestures (Aich et al. 1990). Submissive males respond by fleeing or presenting their hindquarters. In the Smithsonian Channel video[1], male gelada baboons use the lip flip in competition with other males.

Primates also communicate through color. In some species, facial coloration provides information about individual health or status to conspecifics. Mandrills are a good example of this. Female mandrill faces are brighter during ovulation, which may function to communicate reproductive state to males (Setchell et al. 2006). Redness of male mandrill faces is correlated with androgen levels. Thus, facial coloration can, on the one hand, communicate information about competitiveness to other males and information about reproductive fitness to females (Figure 6.27; Setchell et al. 2008). On the other hand, the variation in facial coloration among New World monkeys, ranging from very simple (Figure 6.28a) to highly complex color patterns (Figure 6.28b), appears to be linked to , or the ability to distinguish conspecifics from other species. Species with more complex facial color patterns tend to be those that are sympatric with a larger number of other primate species. In such circumstances, distinct facial coloration and patterning may help individuals recognize conspecifics and reduce the chances of mating with another species (Santana et al. 2012).


Figure 6.28a and 6.28b A ukari displaying bold but simple facial coloration (left). Figure 6.28b A white-bellied spider monkey displaying a complex facial color pattern (right).
Olfactory Communication

All primates use scent to communicate. Females secrete chemicals from their region (the area of the anus and genitals) that provide males with information about their reproductive state. In some species, like macaques and chimpanzees, this olfactory signal is enhanced by a sexual swelling (a visual signal; see Figure 6.24a and discussion above). Olfactory communication is particularly important for New World monkeys, lemurs, and lorises. Male and female squirrel monkeys engage in “urine washing,” in which an individual urinates on its hands and feet and then uses them to spread urine all over its body. The function of urine washing may include marking trails for other group members to follow, self-cleaning or controlling body temperature, dominance displays, enhanced grasping ability while climbing, or communicating reproductive state (Boinski 1992). During aggressive interactions with other males, male ring-tailed lemurs rub their tails with scent from glands on their wrists and chests. They then waive their tails toward their opponent, who responds with his own “stinky tail” display, physical aggression, or by fleeing (Jolly 1966). Both sexes use their anogenital scent glands to mark substrates (like saplings, fallen trees, or tree trunks) in their group’s territory (Jolly 1966). Males also leave visual and scent marks during “spur marking,” in which they impregnate a substrate with scent from their wrist gland after using a thorny “spur” near the gland to cut into it (Figure 6.29). Because substrate marking behavior occurs where the ranges of two groups overlap, and increases during the mating season, the primary function is believed to reinforce territorial boundaries (Mertl-Millhollen 1988).
Tactile Communication
Tactile communication, or touch, is very important in all primate species. Physical contact is used to comfort and reassure, is part of courtship and mating, and is used to establish dominance and alliances. Grooming is an important and clearly enjoyable form of tactile communication for all primates (Figure 6.30a–d). Not only does grooming serve to clean the skin and fur, removing parasites and debris, but it is an important (non-aggressive) behavior that helps reinforce social bonds, repair relationships, and cement alliances. Among chimpanzees, subordinates groom dominants in an effort to receive benefits such as protection, acceptance, and reciprocal grooming. When yellow baboon females engage in more grooming activity (as both givers and receivers), their offspring have an increased chance of surviving to one year (Silk et al. 2003). Although the mechanism behind this relationship is unknown, close associations with group members may provide females and their young offspring with protection from harassment or access to valuable resources that enhance infant survival. Social integration, as exemplified by grooming, is of significant adaptive value to primates.
 |
 |
| Figure 6.30a A group of Japanese macaques grooming each other. | Figure 6.30b Tufted capuchins grooming. |
 |
 |
| Figure 6.30c A group of gelada baboons grooming. | Figure 6.30d Black-and-white ruffed lemurs groom each other. |
It may be surprising in a chapter on nonhuman primates to see a discussion of culture. After all, culture is considered by many, including cultural anthropologists, to be a distinguishing characteristic of humans. Indeed, some anthropologists question claims of culture in primates and other animals. Definitions of animal culture focus on specific behaviors that are unique to one population. Anthropological definitions of human culture emphasize shared ideology (e.g., values, morals, beliefs) and symbols, not just behavior. Using this definition, some cultural anthropologists view primates as lacking culture because of the absence of symbolic life (e.g., religion). However, the longer we study primate groups and populations, the more insight we gain into primate behavioral variation. If we define as the transmission of behavior from one generation to the next through social learning, then we must view at least some of the behavioral variation we see in primates as forms of or a distinctive pattern of behavior shared by multiple individuals in a social group that persists over time (Whitten 2001).
Examples of Culture in Primates
Chimpanzee Culture
Due to both their high level of intelligence and the large number of long-term studies on several different populations, chimpanzees provide the best example of cultural tradition in primates. Research on a variety of animals, including fish, rodents, birds, and monkeys indicate the transmission of a single behavior pattern through social learning, resulting in cultural variation. But chimpanzees, along with orangutans, are the only species other than humans to express cultural variation in multiple behavioral patterns. Examining behavioral variation across chimpanzee study sites, researchers have identified over 40 cultural traditions, or distinct behavioral patterns, in chimpanzees (Whiten 2011). These cultural traditions span the gamut from population-specific prey preferences to tool-use techniques, hunting strategies, and social behaviors.
It is not just the sheer number of cultural traditions that make chimpanzee culture so fascinating. It is that each chimpanzee community displays a unique cultural profile defined by a subset of the known traditions. For example, in Tanzania, chimpanzees fish for termites by poking twigs (which they’ve stripped) into termite mounds. But in Gambia, they use modified twigs to extract honey from holes in trees. In Fongoli, Sénégal, chimpanzees use sticks as “spears” that they stab into tree cavities to hunt for galagos (Figure 6.31). Multiple chimpanzee populations use use a “hammer and anvil” to crack open nuts, but the techniques differ. In some populations, chimps place a nut on a large flat rock and use a stone “hammer” to crack it open; in others, they use pieces of wood. Chimpanzees in Guinea use three stones for nut cracking: one as the anvil, the second one as the hammer, and a third as a wedge to secure the anvil (McGrew 1998). The National Geographic video “Chimps and Tools” (n.d.)[2] provides a glimpse into some of the known variation in chimpanzee tool use. Because the cultural traditions are so diverse and unique, if a researcher can observe enough of a chimpanzee’s behavior, that individual can be assigned to a specific community, much in the same way a human being can be associated with a specific culture based on his or her behavior (Whiten 2011).

So how do chimpanzee cultures develop, and how does cultural transmission occur? Although we do not know for sure how chimpanzee cultural traditions develop initially, it is possible that different groups invent, either accidentally or deliberately, certain behaviors that other individuals copy. There is little evidence currently to support the idea that chimpanzees actively teach one another a new behavior, so it appears that they learn through observation and practice. This lack of teaching is one reason that some primatologists call the traditions in chimpanzees (and other primates) “pre-culture.” However, immigration between communities does appear to be an important avenue of cultural transmission in chimpanzees, much as it is between human cultures. Immigrants (typically females) may bring cultural traditions to their new community, which residents observe and learn. Conversely, immigrants may observe and learn a cultural tradition practiced in their new community (Whiten 2011).
Cultural Transmission in Macaques

Two monkey species are well known for behavioral variation that has been called “pre-cultural” by some primatologists: Japanese macaques and capuchins. The transmission of unique foraging behaviors through a provisioned group of Japanese macaques on Koshima Island is well known (McGrew 1998). In an effort to keep the monkeys nearby, researchers provided them with piles of sweet potatoes. A juvenile female named Imo spontaneously washed a muddy sweet potato in a stream. This new food-processing technique first spread among other juveniles and then gradually to older individuals. Within 30 years, it had spread across generations, and 46 of 57 monkeys in the group engaged in the behavior. Another example comes from a group living far to the north, in the snowy forests of Honshu. Researchers threw apples into hot springs to record the monkeys’ behavior. Not only did the monkeys enter the springs to retrieve the apples, but over multiple years, they learned to immerse themselves in the hot springs to keep warm when not foraging (McGrew 1998; Figure 6.32; watch Japanese macaques using hot springs in the National Geographic video “Meditative Snow Monkeys Hang Out in Hot Springs” (n.d.)[3]. Some primatologists discount the significance of these (pre)cultural traditions since they began as a result of humans providing food to the monkeys and are therefore not “natural” behaviors. However, the behaviors have changed over time, even though the underlying provisioning either did not change or ceased altogether (McGrew 1998). For example, although sweet potato washing started in freshwater, it gradually shifted to seawater, apparently to add salt for flavor. Thirty years after the behavior started, the most common form involved dipping the potato into salt water, even if it was clean. Similarly, female macaques entering the hot springs initially left their young infants at the edge, but today juveniles play and even swim underwater in the hot springs. These examples share several characteristics with human culture, including invention or modification of behavior, transmission of behavior between individuals, and the persistence of the behavior across generations (McGrew 1998).
CONCLUSION
Primates are socially complex and extremely intelligent. Highly adaptable, they display significant variation in diet, habitat, and behavior. By studying primates in their natural habitats, we learn how their behavior and morphology are influenced by ecology, including the foods they eat and the other species with which they live. As our closest living relatives, primates provide important insights into the evolution of human social behavior, language, and culture. These are topics you will learn about in later chapters of this book.
Review Questions
- If anthropology is the study of humans, why do some anthropologists study primates?
- What is the nature of interactions between primates and other members of their broader ecological communities, including other species of primates?
- What is the difference between a social system and a mating system? Describe the variety of social and mating systems observed in primates. How do primatologists use the distribution of food, females, and males to understand this variation, including the fact that two species can have the same mating system but different social systems? Compare and contrast male and female mating strategies. Why and how, do females choose a potential mate? Why and how do males compete for potential mates?
- What are the costs and benefits of group living? If living in a group is beneficial for most primates, why do some individuals disperse and leave their group? How do the costs and benefits of dispersal differ for males and females?
- Discuss the variation in primate communication. How is communication between primates similar to and different from communication between humans?
- What is the evidence for cultural variation in primates? How do primatologists think cultural transmission occurs in primates? How do you think this process compares to cultural transmission in humans?
Key Terms
Abundance: How much food is available in a given area.
Affiliative: A description of non-aggressive social interactions and associations between individuals.
Allopatric: Two or more species that do not overlap in geographic distribution.
Anogenital: Relating to the anus and genitals.
Basal metabolic rate: The rate at which an individual uses energy when at rest.
Carnivores: Organisms whose diet consists primarily of animal tissue.
Coalition: A temporary group composed of two or more individuals who work together to achieve a common goal. It is often used in reference to male-male competition, such as when two less-competitive males join forces against a more-competitive male.
Competitive exclusion principle: The idea that two species that compete for the exact same resources cannot coexist.
Conspecifics: Members of the same species.
Culture: The transmission of behavior from one generation to the next through observation and imitation.
Cultural tradition: A distinctive pattern of behavior shared by multiple individuals in a social group, which persists over time and is acquired through social learning.
Crypsis: The ability to avoid detection by other organisms.
Day-range length: The distance traveled in a day.
Direct fitness: An individual’s genetic contribution to future generations that is due to offspring production.
Dispersal: To leave one’s group or area. This may or may not involve entering another group.
Distribution: How food is spread out.
Dominance hierarchy: The ranked organization of individuals established by the outcome of aggressive-submissive interactions.
Feeding: The act of consuming food.
Fission-fusion: Societies in which group composition is flexible, such as chimpanzee and spider monkey societies. Individuals may break up into smaller feeding groups (fission) and combine into larger groups (fusion).
Fitness: An individual’s reproductive success relative to other members of the same species.
Foraging: The act of searching for food.
Folivores: Organisms whose diet consists primarily of leaves.
Frugivores: Organisms whose diet consists primarily of fruit.
Home range: The area a group or individual uses over a given period of time (often over a year).
Inbreeding: Reproduction between relatives.
Inbreeding depression: Harmful genetic effects of breeding between relatives.
Indirect fitness: An individual’s genetic contribution to future generations that is due to the reproduction of non-descent relatives.
Infanticide: The killing of infants of one’s own species.
Insectivores: Organisms whose diets consist primarily of insects.
Intrasexual selection: Selection for traits that enhance the ability of members of one sex to compete amongst themselves.
Interbirth interval: The length of time between successive births.
Intersexual selection: The selection for traits that enhance the ability of the members of one sex to attract the attention of the other.
Mating system: A way of describing which male(s) and female(s) mate.
Mobbing: Cooperatively attacking or harassing a predator.
Monogamous: A mating system in which one male mates with one female.
Natal group: The group into which an organism is born.
Natal dispersal: Emigrating from the group into which one is born.
Niche: The role of a species in its environment; how it meets its needs for food, shelter, etc.
Niche partitioning: The process by which potentially competing species reduce competition by using the environment differently.
Omnivores: Organisms whose diet consists of plant and animal matter.
Operational sex ratio: The ratio of sexually active (or available) males to sexually active (or available) females.
Parental investment: Any time or energy a parent devotes to the current offspring that enhances its survival (and eventual reproductive success) at the expense of the parent’s ability to invest in the next offspring.
Paternity certainty: Confidence in which male fathered an offspring.
Paternity confusion: When males are uncertain if they fathered an offspring. This is often a female strategy to reduce the chance of infanticide.
Philopatric: Remaining in the group of one’s birth.
Piloerection: Raising one’s hair or fur in an effort to look bigger.
Polyandry: A mating system in which multiple males mate with a single breeding female.
Polygamy: A mating system in which multiple males mate with multiple females.
Polygyny: A mating system in which one male mates with multiple females.
Polyspecific associations: Associations between two or more different species involving behavioral changes by at least one of the associated species.
Primate community: All living organisms that occur in an area that includes primates.
Primatologist: A scientist who studies primate behavior and/or ecology.
Primatology: The scientific field that studies primate behavior and/or ecology.
Receptive: A term used to describe females who are ready for sexual reproduction (i.e., not pregnant or nursing).
Reproductive success: An individual’s genetic contribution to future generations.
Reproductive suppression: The prevention or inhibition of reproduction of healthy adults.
Secondary sexual characteristics: Characteristics that appear at time of sexual maturity. These are not directly involved in reproduction, but they provide individuals an advantage in courtship and competition for mates.
Semantic communication: The systematic use of signals to refer to objects in the environment.
Sexual dimorphism: When males and females of a species have different morphological traits.
Sexual monomorphism: When males and females of a species have similar morphological traits.
Sexual selection: The selection for traits that increase mating success. This occurs via intersexual selection and intrasexual selection.
Sexual swelling: Area of the hindquarters that change in size, shape and often color over the course of a female’s reproductive cycle, reaching maximum size at ovulation. Occurs in many Old World primate species.
Social learning: The idea that new behaviors can be acquired by observing and imitating others.
Social system: A way of describing the typical number of males and females of all age classes that live together.
Solitary: Living alone.
Species recognition: Recognition of conspecifics.
Sperm competition: Competition between sperm of two or more different males to fertilize the same egg.
Sympatric: Two or more species that overlap in geographic distribution.
Territory: A home range whose boundary is defended from intrusion by conspecifics.
Vertebrates: The group of animals characterized by an internal spinal column or backbone. This includes fish, amphibians, reptiles, birds, and mammals.
Vigilance: Watchful behavior to detect or in response to potential danger, usually in the form of predators or potential competitors.
About the Author
Karin Enstam Jaffe
Sonoma State University, karin.jaffe@sonoma.edu

Dr. Karin Enstam Jaffe has loved primates since she was five years old. As an undergraduate at U.C. San Diego, she participated in projects studying orangutans, langurs, and Mona monkeys. She earned her Ph.D. in Anthropology from U.C. Davis studying vervet and patas monkey anti-predator behavior. Dr. Jaffe has over 20 years of experience studying primate behavior in Kenya and Grenada and at the San Diego Zoo, San Francisco Zoo, and Safari West Wildlife Preserve in Sonoma County, California. She has been a faculty member in the Anthropology Department at Sonoma State University since August 2002. A dedicated teacher-scholar, Dr. Jaffe has won several teaching, scholarship, and mentoring awards, including SSU’s Excellence in Teaching Award, Educational Experience Enhancement Award, and the President’s Excellence in Scholarship Award. In addition to teaching, she is a Research Associate at Oakland Zoo, where she has been involved in behavioral enrichment research involving ring-tailed lemurs, chimpanzees, and sun bears, as well as a study of the social network of hamadryas baboons.
For Further Exploration
Fossey, Dian. 1983. Gorillas in the Mist. London: Hodder & Stoughton.
Goodall, Jane. 1971. In the Shadow of Man. Boston: Houghton Mifflin.
Rowe, Noel, and Marc Myers, eds. 2016. All the World’s Primates. Charleston, Rhode Island: Pogonias Press.
Strier, Karen B. 2017. Primate Behavioral Ecology. 5th ed. New York: Routledge.
Primate Info Net (http://pin.primate.wisc.edu/) is an information service of the National Primate Research Center at the University of Wisconsin, Madison. It includes Primate Factsheets, primate news and publications, a list of primate-related jobs, and an international directory of primatology, among other information.
Primate Specialist Group (http://www.primate-sg.org/) is a collection of scientists and conservationists who work in dozens of African, Asian, and Latin American nations to promote research on primate conservation.
References
Aich, H., R. Moos-Heilen, and E. Zimmermann. 1990. “Vocalizations of Adult Gelada Baboons (Theropithecus gelada): Acoustic Structure and Behavioural Context.” Folia Primatologica 55 (3–4): 109–132.
Arlet, Małgorzata E., Freerk Molleman, and Colin Chapman. 2007. “Indications for Female Mate Choice in Grey-Cheeked Mangabeys Lophocebus albigena johnstoni in Kibale National Park, Uganda.” Acta Ethologica 10 (2): 89–95.
Baker, Andrew J., James M. Dietz, and Devra G. Kleiman. 1993. “Behavioural Evidence for Monopolization of Paternity in Multi-Male Groups of Golden Lion Tamarins.” Animal Behaviour 46 (6): 1,091–1,103.
Bell, Sarah A. 2017. “Galdikas, Birute.” In The International Encyclopedia of Primatology, Volume A–G, edited by Agustín Fuentes, 445–446. Malden, Massachusetts: John Wiley & Sons.
Boesch, Christophe. 2002. “Cooperative Hunting Roles Among Taï Chimpanzees.” Human Nature 13 (1): 27–46.
Boesch, Christophe, and Hedwige Boesch. 1989. “Hunting Behavior of Wild Chimpanzees in the Taï National Park.” American Journal of Physical Anthropology 78 (4): 547–573.
Boinski, S. 1992. “Olfactory Communication among Costa Rican Squirrel Monkeys: A Field Study.” Folia Primatologica 59 (3): 127–136.
Borries, Carola, Kristin Launhardt, Cornelia Epplen, Jörg T. Epplen, and Paul Winkler. 1999. “Males as Infant Protectors in Hanuman Langurs (Presbytis entellus) Living in Multimale Groups: Defence Pattern, Paternity, and Sexual Behaviour.” Behavioral Ecology and Sociobiology 46 (5): 350–356.
Chapman, Colin A., and Carlos A. Peres. 2001. “Primate Conservation in the New Millennium: The Role of Scientists.” Evolutionary Anthropology: Issues, News, and Reviews 10 (1): 16–33.
Cheney, D. L., and R. M. Seyfarth. 1987. “The Influence of Intergroup Competition on the Survival and Reproduction of Female Vervet Monkeys.” Behavioral Ecology and Sociobiology 21 (6): 375–386.
Chism, J. B., T. E. Rowell, and D. Olson. 1984. “Life History Patterns of Female Patas Monkeys.” In Female Primates: Studies of Women Primatologists, edited by Meredith Small, 175–190. New York: A.R. Liss.
Clarke, Margaret R., Kenneth E. Glander, and Evan L. Zucker. 1998. “Infant-Nonmother Interactions in Free-Ranging Howlers (Alouatta palliata) in Costa Rica. International Journal of Primatology 19 (3): 451–472.
Crockett, Carolyn M., and Theresa Pope. 1988. “Inferring Patterns of Aggression from Red Howler Monkey Injuries.” American Journal of Primatology 15 (4): 289–308.
Díaz-Muñoz, Samuel L. 2011. “Paternity and Relatedness in a Polyandrous Nonhuman Primate: Testing Adaptive Hypotheses of Male Reproductive Cooperation.” Animal Behaviour 82 (3): 563–571.
Digby, Leslie J., Stephen F. Ferrari, and Wendy Saltzman. (2007) 2011. “Callitrichines: The Role of Competition in Cooperatively Breeding Species.” In Primates in Perspective, edited by Christina J. Campbell, AugustÍn Fuentes, Katherine C. MacKinnon, Simon K. Bearder, and Rebecca M. Stumpf, 91–10. Second edition, New York: Oxford University Press.
Eckardt, Winnie, and Klaus Zuberbühler. 2004. “Cooperation and Competition in Two Forest Monkeys.” Behavioral Ecology 15 (3): 400–411.
Enstam, Karin L., and Lynne A. Isbell. 2002. “Comparison of Responses to Alarm Calls by Patas (Erythrocebus patas) and Vervet (Cercopithecus aethiops) Monkeys in Relation to Habitat Structure.” American Journal of Physical Anthropology 119 (1): 3–14.
Enstam, Karin L., Lynne A. Isbell, and Thomas W. de Maar. 2002. “Male Demography, Female Mating Behavior, and Infanticide in Wild Patas Monkeys (Erythrocebus patas).” International Journal of Primatology. 23 (1): 85–104.
Fedigan, Linda Marie. 1990. “Vertebrate Predation in Cebus capucinus: Meat Eating in a Neotropical Monkey.” Folia Primatologica 54 (3–4): 196–205.
Fischer, Julia, Kurt Hammerschmidt, Dorothy L. Cheney, and Robert M. Seyfarth. 2008. “Acoustic Features of Female Chacma Baboon Barks.” Ethology 107 (1): 33–54.
Fossey, Dian. 1983. Gorillas in the Mist. London: Hodder & Stoughton.
Gardner, Charlie J., Patrick Radolalaina, Mahandry Rajerison, and Harry W. Greene. 2015. “Cooperative Rescue and Predator Fatality Involving a Group-Living Strepsirrhine, Coquerel’s Sifaka (Propithecus coquereli), and a Madagascar Ground Boa (Acrantophis madagascariensis).” Primates 56 (2): 127–129.
Gautier-Hion, Annie, René Quris, and Jean-Pierre Gautier. 1983. “Monospecific vs. Polyspecific Life: A Comparative Study of Foraging and Antipredator Tactics in a Community of Cercopithecus Monkeys.” Behavioral Ecology and Sociobiology 12 (4): 325–335.
Harcourt, A. H., P. H. Harvey, S. G. Larson, and R. V. Short. 1981. “Testis Weight, Body Weight and Breeding System in Primates.” Nature 293 (5,827): 55–57.
Henzi, Peter, and Louise Barrett. 2003. “Evolutionary Ecology, Sexual Conflict, and Behavioral Differentiation among Baboon Populations.” Evolutionary Anthropology 12 (5): 217–230.
Isbell, Lynne A. 1991. “Contest and Scramble Competition: Patterns of Female Aggression and Ranging Behavior Among Primates.” Behavioral Ecology 2 (2): 143–155.
Isbell, Lynne A., Dorothy L. Cheney, and Robert M. Seyfarth. 1990. “Costs and Benefits of Home Range Shifts Among Vervet Monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya.” Behavioral Ecology and Sociobiology 27 (5): 351–358.
Isbell, Lynne A., and Dirk Van Vuren. 1996. “Differential Costs of Locational and Social Dispersal and Their Consequences for Female Group-Living Primates.” Behaviour 133 (1–2): 1–36.
Jolly, Alison. 1966. Lemur Behavior: A Madagascar Field Study. Chicago: University of Chicago Press.
Lutz, Clayton L., Duane R. Diefenbach, and Christopher S. Rosenberry. 2016. “Proximate Influences on Female Dispersal in White-Tailed Deer.” Journal of Wildlife Management 80 (7): 1,218–1,226.
Marty, Pascal R., Maria A. van Noordwijk, Michael Heistermann, Erik P. Willems, Lynda P. Dunkel, Manuela Cadilek, Muhammad Agil, and Tony Weingrill. 2015. “Endocrinological Correlates of Male Bimaturism in Wild Bornean Orangutans.” American Journal of Primatology 77 (11): 1,170–1,178.
McGrew, W. C. 1998. “Culture in Nonhuman Primates?” Annual Review of Anthropology 27 (1): 301–328.
Mertl-Millhollen, Anne S. 1988. “Olfactory Demarcation of Territorial but Not Home Range Boundaries by Lemur catta.” Folia Primatologica 50 (3–4): 175–187.
National Geographic. n.d. “Chimps and Tools.” Accessed July 25, 2019. https://video.nationalgeographic.com/video/00000144-0a1e-d3cb-a96c-7b1fadbd0000
National Geographic. n.d. “Meditative Snow Monkeys Hang Out in Hot Springs.” Accessed July 25, 2019. https://video.nationalgeographic.com/video/short-film-showcase/00000149-d415-de71-a9eb-dc9539210000
Nekaris, K. Anne-Isola, Richard S. Moore, E. Johanna Rode, and Bryan G. Fry. 2013. “Mad, Bad, and Dangerous to Know: The Biochemistry, Ecology, and Evolution of Slow Loris Venom.” The Journal of Venomous Animals and Toxins Including Tropical Diseases 19 (1): 21.
Peres, Carlosa. 1992. “Prey-Capture Benefits in a Mixed-Species Group of Amazonian Tamarins, Saguinus fuscicollis and S. mystax.” Behavioral Ecology and Sociobiology 31 (5): 339–347. https://doi.org/10.1007/bf00177774.
Raboy, Becky E., Gustavo R. Canale, and James M. Dietz. 2008. “Ecology of Callithrix kuhlii and a Review of Eastern Brazilian Marmosets.” International Journal of Primatology 29 (2): 449–467.
Ransom, Timothy W. 1981. Beach Troop of the Gombe. East Brunswick, New Jersey: Bucknell University Press.
Santana, Sharlene E., Jessica Lynch Alfaro, and Michael E. Alfaro. 2012. “Adaptive Evolution of Facial Colour Patterns in Neotropical Primates.” Proceedings of the Royal Society B: Biological Sciences 279 (1,736): 2,204–2,211.
Schön Ybarra, M. A. 1986. “Loud Calls of Adult Male Red Howling Monkeys (Alouatta seniculus).” Folia Primatologica 47 (4): 204–216.
Schwitzer, Christoph, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis, and Alison Cotton. 2017. Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. Arlington, VA: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society.
Sekulic, Ranka. 1982. “The Function of Howling in Red Howler Monkeys (Alouatta seniculus).” Behaviour 81 (1): 38–54.
Setchell, Joanna M., Tessa Smith, E. Jean Wickings, and Leslie A. Knapp. 2008. “Social Correlates of Testosterone and Ornamentation in Male Mandrills.” Hormones and Behavior 54 (3): 365–372.
Setchell, Joanna M., E. Jean Wickings, and Leslie A. Knapp. 2006. “Signal Content of Red Facial Coloration in Female Mandrills (Mandrillus sphinx).” Proceedings of the Royal Society B: Biological Sciences 273 (1599): 2395–2400.
Seyfarth, R. M., D. L. Cheney, and P. Marler. 1980a. “Monkey Responses to Three Different Alarm Calls: Evidence of Predator Classification and Semantic Communication.” Science 210 (4,471): 801–803.
Seyfarth, Robert M., Dorothy L. Cheney, and Peter Marler. 1980b. “Vervet Monkey Alarm Calls: Semantic Communication in a Free-Ranging Primate.” Animal Behaviour 28 (4): 1,070–1,094.
Silk, Joan B., Susan C. Alberts, and Jeanne Altmann. 2003. “Social Bonds of Female Baboons Enhance Infant Survival.” Science 302 (5,648): 1,231–1,234.
Smith, David Glenn. 1994. “Male Dominance and Reproductive Success in a Captive Group of Rhesus Macaques (Macaca mulatta).” Behaviour 129 (3): 225–242.
Smithsonian Channel. June 9, 2017. “Why These Vegetarian Monkeys Have Sharp Predator Teeth.” Accessed July 25, 2019. https://www.youtube.com/watch?time_continue=145&v=aC6iYj_EBjY
Stanford, Craig B. 2017. “Goodall, Jane.” In The International Encyclopedia of Primatology, Volume A–G, edited by Agustín Fuentes, 471–472. Malden, Massachusetts: John Wiley & Sons.
Stewart, Kelly. 2017. “Fossey, Dian.” In The International Encyclopedia of Primatology, Volume A–G, edited by Agustín Fuentes, 432–433. Malden, Massachusetts: John Wiley & Sons.
Stumpf, R. M., M. Emery Thompson, M. N. Muller, and R. W. Wrangham. 2009. “The Context of Female Dispersal in Kanyawara Chimpanzees.” Behaviour 146 (4–5): 629–656.
Swedell, Larissa, and Amy Schreier. 2006. “Male Aggression toward Females in Hamadryas Baboons: Conditioning, Coercion, and Control.” In Sexual Coercion in Primates and Humans, edited by Martin N. Muller and Richard W. Wrangham, 244–268. Cambridge, Massachusetts: Harvard University Press.
Terborgh, John. 1984. Five New World Primates: A Study in Comparative Ecology. Princeton: Princeton University Press.
Trivers, Robert L. 1972. “Parental Investment and Sexual Selection.” In Sexual Selection and the Descent of Man, 1871–1971, edited by Bernard Campbell, 136–179. Chicago: Aldine.
van Schaik, Carel P., and Peter M. Kappeler. 1997. “Infanticide Risk and the Evolution of Male-Female Association in Primates.” Proceedings of the Royal Society B: Biological Sciences 264 (1,388): 1,687–1,694.
Whiten, Andrew. 2011. “The Scope of Culture in Chimpanzees, Humans and Ancestral Apes.” Philosophical Transactions of the Royal Society of London B: Biological Sciences 366 (1,567): 997–1,007.
Whitten, Patricia L. 1983. “Diet and Dominance among Female Vervet Monkeys (Cercopithecus aethiops).” American Journal of Primatology 5 (2): 139–159.
Wiens, Frank, and Annette Zitzmann. 2003. “Social Structure of the Solitary Slow Loris Nycticebus coucang (Lorisidae).” Journal of Zoology 261 (1): 35–46.
Zuberbühler, Klaus, David Jenny, and Redouan Bshary. 1999. “The Predator Deterrence Function of Primate Alarm Calls.” Ethology 105 (6): 477–490.
Zuberbühler, Klaus, Ronald Noë, and Robert M. Seyfarth. 1997. “Diana Monkey Long-Distance Calls: Messages for Conspecifics and Predators.” Animal Behaviour 53 (3): 589–604.
Acknowledgements
The author is grateful to the editors for the opportunity to contribute to this open-source textbook. She thanks Stephanie Etting for her encouragement and support during the writing of this chapter. Her suggestions, along with comments made by two anonymous reviewers on an earlier version of this chapter, improved the final version considerably. Finally, she thanks all the primatologists who came before her, especially her advisor, Lynne A. Isbell, for their tireless efforts to understand the behavior and ecology of the living primates. Without their work, this chapter would not have been possible.
Figure Attributions
Figure 6.1a Snow monkey baby milk time by Daisuke tashiro is used under a CC BY-SA 2.0 License.
Figure 6.1b Stone tool use by a capuchin monkey by Tiago Falótico is used under a CC BY-SA 4.0 License.
Figure 6.2 Laikipia location map by Nairobi123 is used under a CC BY-SA 3.0 License.
Figure 6.3a Female patas monkey with infant by Karin Enstam Jaffe is under a CC BY-NC 4.0 License.
Figure 6.3b Patas habitat in Laikipia, Kenya by Karin Enstam Jaffe is under a CC BY-NC 4.0 License.
Figure 6.3a Monkey (27996706462) by Martie Swart from Johannesburg, South Africa is under a CC BY 2.0 License.
Figure 6.4b Vervet habitat in Laikipia, Kenya by Karin Enstam Jaffe is under a CC BY-NC 4.0 License.
Figure 6.5 Karin Enstam Jaffe observing patas monkeys in Laikipia, Kenya by Rebecca Chancellor is under a CC BY-NC 4.0 License.
Figure 6.6a Spectral Tarsier Tarsius tarsier (7911549768) by Bernard DUPONT from FRANCE has been modified (cropped) and is used under a CC BY-SA 2.0 License.
Figure 6.6b Mountain gorilla (Gorilla beringei beringei) eating by Charles J Sharp creator QS:P170,Q54800218 is used under a CC BY-SA 4.0 License.
Figure 6.7 Food abundance and food scarcity by Karin Enstam Jaffe original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
Figure 6.8 Food distribution patterns by Karin Enstam Jaffe original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
Figure 6.9 Food distribution patterns of folivores, frugivores, and insectivores a derivative work by Karin Enstam Jaffe original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License. [Includes Green fruit tree, Lemon tree, and Ladybug image, all from publicdomainvectors.org designated to the public domain (CC0)].
Figure 6.10 Termite mound-Tanzania by Vierka Maráková, Slovakia (Krokodild) has been designated to the public domain (CC0).
Figure 6.11a Patas Monkey Jr by alex roberts from San Francisco, USA is used under a CC BY-SA 2.0 License.
Figure 6.11b Chlorocebus pygerythrus by Xlandfair at English Wikipedia has been designated to the public domain (CC0).
Figure 6.11c Male Olive Baboon 2 by ryan harvey from Portland, OR is used under a CC BY-SA 2.0 License.
Figure 6.11d Komba ušatá2 by Petr Hamerník is used under a CC BY-SA 4.0 License.
Figure 6.12 Muriquis 3 by Mônica Imbuzeiro is used under a CC BY-SA 4.0 License.
Figure 6.13 Examples of primate communities table original to Explorations: An Open Invitation to Biological Anthropology by Karin Enstam Jaffe is under a CC BY-NC 4.0 License.
Figure 6.14a Lion tailed macaque eating Lizard by Prasadchangarath is used under a CC BY-SA 4.0 License.
Figure 6.14b Gombe Stream NP Beute by Ikiwaner is used under a GNU License.
Figure 6.15a Leopard africa by JanErkamp at the English Wikipedia is used under a CC BY-SA 3.0 License.
Figure 6.15b Jaguar (Panthera onca palustris) male Rio Negro 2 by Charles J Sharp creator QS:P170,Q54800218 is used under a CC BY-SA 4.0 License.
Figure 6.15c Male Tiger Ranthambhore (3646793728) by Koshy Koshy is used under a CC BY 2.0 License.
Figure 6.15d Fosa fotovideo by Bertal is used under a CC BY-SA 3.0 License.
Figure 6.15e Martial eagle (Polemaetus bellicosus) by Charles J Sharp creator QS:P170,Q54800218 is used under a CC BY-SA 4.0 License.
Figure 6.15f Harpy Eagle with wings lifted by Jonathan Wilkins is used under a CC BY-SA 3.0 License.
Figure 6.15g Python natalensis G. J. Alexander by Graham J. Alexander, University of the Witwatersrand has been designated to the public domain (CC0).
Figure 6.16 Slow Loris by Jmiksanek is used under a CC BY-SA 3.0 License.
Figure 6.17 Monkey group by Natureloverperson is used under a CC BY-SA 4.0 License.
Figure 6.18a Deforestation near Bukit Tiga Puluh NP by Aidenvironment (1998) is used under a CC BY-SA 2.0 License.
Figure 6.18b Lemur poaching 004 by author who does not wish to be named for safety reasons is used under a CC BY-SA 3.0 License.
Figure 6.19a Dierenpark Emmen baboon (2679944324) by robin bos from heerenveen, the Netherlands is used under a CC BY 2.0 License.
Figure 6.19b Male Gelada Baboon, Chenek, Simien Mountains (6190375633) by Rod Waddington from Kergunyah, Australia has been modified (cropped) and is used under a CC BY-SA 2.0 License.
Figure 6.20a Azy (orangutan) at the Indianapolis Zoo by Tom Britt is used under a CC BY 2.0 License.
Figure 6.20b Orangutan male (15433476577) by Russ is used under a CC BY 2.0 License.
Figure 6.21a Emperor Tamarin with babies sf by Brocken Inaglory is used under a CC BY-SA 3.0 License.
Figure 6.21b Family of Common Marmoset – REGUA – Brazil MG 9480 (12930855765) by Francesco Veronesi from Italy is used under a CC BY-SA 2.0 License.
Figure 6.22 Social and Mating Systems by Karin Enstam Jaffe original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
Figure 6.23a Jane Goodall HK by Jeekc is used under a CC BY-SA 3.0 License.
Figure 6.23b US-223658 Dian Fossey (2925879356) by Mary-Lynn is used under a CC BY 2.0 License.
Figure 6.23c Dr Birute Galdikas by Simon Fraser University – University Communications is used under a CC BY 2.0 License.
Figure 6.24a Sexual swelling in female Hamadryas baboon by Mamoritai is used under a CC BY-SA 2.0 License.
Figure 6.24b Hamadryas baboon at Giza Zoo by Hatem Moushir 36 by Hatem Moushir is used under a CC BY-SA 3.0 License.
Figure 6.25 Mandrill 08 by SuperJew is used under a CC BY-SA 4.0 License.
Figure 6.26 BabouinGeladaAuReveil by BluesyPete is used under a CC BY-SA 3.0 License.
Figure 6.27 Mandrill 09 by SuperJew is used under a CC BY-SA 4.0 License.
Figure 6.28a Uakari by Coada dragos is used under a CC BY-SA 4.0 License.
Figure 6.28b Ateles belzebuth (White-bellied spider monkey) 2 by Ewa is used under a CC BY 2.0 License.
Figure 6.29 Lemur catta 004 by Alex Dunkel (Visionholder) is used under a CC BY-SA 3.0 License.
Figure 6.30a Yakushima macaques grooming each other by Grendelkhan is used under a CC BY-SA 4.0 License.
Figure 6.30b Tufted capuchin monkeys grooming session III by Adrian Soldati is used under a CC BY-SA 4.0 License.
Figure 6.30c Baboons Wunania 012018 by Kim Toogood is used under a CC BY-SA 4.0 License.
Figure 6.30d Black-and-white ruffed lemur 03 by Mattis2412 has been designated to the public domain (CC0 1.0).
Figure 6.31 Pan troglodytes, tool use in Senegal by J. D. Pruetz, P. Bertolani, K. Boyer Ontl, S. Lindshield, M. Shelley, and E. G. Wessling is used under a CC BY 4.0 License.
Figure 6.32 Jigokudani hotspring in Nagano Japan 001 by Yosemite is used under a CC BY-SA 3.0 License.
- Smithsonian Channel. June 9, 2017. “Why These Vegetarian Monkeys Have Sharp Predator Teeth.” Accessed July 25, 2019. https://www.youtube.com/watch?time_continue=145&v=aC6iYj_EBjY ↵
- National Geographic. n.d. “Chimps and Tools.” Accessed July 25, 2019. https://video.nationalgeographic.com/video/00000144-0a1e-d3cb-a96c-7b1fadbd0000 ↵
- National Geographic. n.d. “Meditative Snow Monkeys Hang Out in Hot Springs.” Accessed July 25, 2019. https://video.nationalgeographic.com/video/short-film-showcase/00000149-d415-de71-a9eb-dc9539210000 ↵
The scientific field that studies primate behavior and/or ecology.
A scientist who studies primate behavior and/or ecology.
Living alone.
The act of searching for food.
The act of consuming food.
Organisms whose diet consists of plant and animal matter.
Having a diet consisting primarily of fruit.
Having a diet consisting primarily of leaves.
Organisms whose diets consist primarily of insects.
How much food is available in a given area.
How food is spread out.
The rate at which an individual uses energy when at rest.
The area a group or individual uses over a given period of time (often over a year).
A home range whose boundary is defended from intrusion by conspecifics.
The distance traveled in a day.
The ranked organization of individuals established by the outcome of aggressive-submissive interactions.
Members of the same species.
Two or more species that overlap in geographic distribution.
All living organisms that occur in an area that includes primates.
Two or more species that do not overlap in geographic distribution.
The idea that two species that compete for the exact same resources cannot coexist.
The role of a species in its environment; how it meets its needs for food, shelter, etc.
The process by which potentially competing species reduce competition by using the environment differently.
The group of animals characterized by an internal spinal column or backbone. This includes fish, amphibians, reptiles, birds, and mammals.
Organisms whose diet consists primarily of animal tissue.
The ability to avoid detection by other organisms.
To leave one’s group or area. This may or may not involve entering another group.
Societies in which group composition is flexible, such as chimpanzee and spider monkey societies. Individuals may break up into smaller feeding groups (fission) and combine into larger groups (fusion).
An individual’s reproductive success relative to other members of the same species
The length of time between successive births.
Watchful behavior to detect or in response to potential danger, usually in the form of predators or potential competitors.
Cooperatively attacking or harassing a predator.
Associations between two or more different species involving behavioral changes by at least one of the associated species.
Remaining in the group of one’s birth.
A term used to describe females who are ready for sexual reproduction (i.e., not pregnant or nursing).
The group into which an organism is born.
An individual’s genetic contribution to future generations.
The selection of mates exclusively from within a small, closed population.
Harmful genetic effects of breeding between relatives.
Emigrating from the group into which one is born.
Any time or energy a parent devotes to the current offspring that enhances its survival (and eventual reproductive success) at the expense of the parent’s ability to invest in the next offspring.
The idea that new behaviors can be acquired by observing and imitating others.
The killing of infants of one’s own species.
Confidence in which male fathered an offspring.
An aspect of natural selection in which the selective pressure specifically affects reproductive success (the ability to successfully breed and raise offspring).
Selection for traits that enhance the ability of members of one sex to compete amongst themselves.
The selection for traits that enhance the ability of the members of one sex to attract the attention of the other.
The ratio of sexually active (or available) males to sexually active (or available) females.
When males and females of a species have different morphological traits.
A temporary group composed of two or more individuals who work together to achieve a common goal. It is often used in reference to male-male competition, such as when two less-competitive males join forces against a more-competitive male.
Competition between sperm of two or more different males to fertilize the same egg.
Characteristics that appear at time of sexual maturity. These are not directly involved in reproduction, but they provide individuals an advantage in courtship and competition for mates.
The prevention or inhibition of reproduction of healthy adults.
An individual’s genetic contribution to future generations that is due to the reproduction of non-descent relatives.
An individual’s genetic contribution to future generations that is due to offspring production.
When males are uncertain if they fathered an offspring. This is often a female strategy to reduce the chance of infanticide.
A way of describing the typical number of males and females of all age classes that live together.
A way of describing which male(s) and female(s) mate.
A mating system in which one male mates with multiple females.
A mating system in which one male mates with one female.
When males and females of a species have similar morphological traits.
A mating system in which multiple males mate with multiple females.
A mating system in which multiple males mate with a single breeding female.
The systematic use of signals to refer to objects in the environment.
Raising one’s hair or fur in an effort to look bigger.
Area of the hindquarters that change in size, shape and often color over the course of a female’s reproductive cycle, reaching maximum size at ovulation. Occurs in many Old World primate species.
Recognition of conspecifics.
Relating to the anus and genitals.
A description of non-aggressive social interactions and associations between individuals.
The transmission of behavior from one generation to the next through observation and imitation.
A distinctive pattern of behavior shared by multiple individuals in a social group, which persists over time and is acquired through social learning.
