Private: Main Body
10 Early Members of the Genus Homo
Bonnie Yoshida-Levine Ph.D., Grossmont College
Learning Objectives
- Describe how early Pleistocene climate change influenced the evolution of the genus Homo.
- Identify the characteristics that define the genus Homo.
- Describe the skeletal anatomy of Homo habilis and Homo erectus based on the fossil evidence.
- Assess opposing points of view about how early Homo should be classified.
- Describe what is known about the adaptive strategies of early members of the Homo genus, including tool technologies, diet, migration patterns, and other behavioral trends.
The boy was no older than 9 when he perished by the swampy shores of the lake. After death, his slender, long-limbed body sank into the mud of the lake shallows. His bones fossilized and lay undisturbed for 1.5 million years. In the 1980s, fossil hunter Kimoya Kimeu, working on the western shore of Lake Turkana, Kenya, glimpsed a dark colored piece of bone eroding in a hillside. This small skull fragment led to the discovery of what is arguably the world’s most complete early hominin fossil—a youth identified as a member of the species Homo erectus. Now known as Nariokotome Boy, after the nearby lake village, the skeleton has provided a wealth of information about the early evolution of our own genus, Homo (see Figure 10.1). Today, a stone monument with an inscription in three languages—English, Swahili, and the local Turkana language—marks the site of this momentous fossil discovery.


Figure 10.1 Skeleton of a young male Homo erectus known as “Nariokotome Boy,” along with an artist’s depiction of how he may have looked during his life. This is the most complete hominin fossil from this time period ever found.
The previous chapter described our oldest human ancestors, primarily members of the genus Australopithecus who lived between 2 million and 4 million years ago. This chapter introduces the earliest members of the genus Homo, focusing on the species Homo habilis and Homo erectus.
DEFINING THE GENUS HOMO
Since our discipline is fundamentally concerned with what makes us human, defining our own genus takes on special significance for anthropologists. The genus is the next level up from species in the classification system originally devised by Carolus Linnaeus. In the 1758 publication Systema Naturae, Linnaeus assigned humans the genus name Homo, meaning “person.” Under this classification scheme, Linnaeus included several ape species, as well as wild children and mythical humans such as cave-dwelling troglodytes. In the present-day classification, the apes and monster people have long been removed, and our species, Homo sapiens, remains as its only living representative. But ever since scientists have acknowledged the existence of extinct species of humans, they have debated which of these display sufficient “humanness” to merit classification in our genus.
When grouping species into a common genus, biologists will consider criteria such as physical characteristics (morphology), evidence of recent common ancestry, and adaptive strategy (use of the environment). However, there is disagreement about which of those criteria should be prioritized, as well as how specific fossils should be interpreted in light of the criteria.
There is general agreement that species classified as Homo should share characteristics broadly similar to our species. These include the following:
- a relatively large brain size, indicating a high degree of intelligence;
- a smaller and flatter face;
- smaller jaws and teeth; and
- increased reliance on culture, particularly the use of stone tools, to exploit a greater diversity of environments (adaptive zone).
Some researchers would include larger overall body size and limb proportions (longer legs/shorter arms) in this list. There is also an apparent decline in sexual dimorphism (body-size differences between males and females). While these criteria seem relatively clear-cut, evaluating them in the fossil record has proved more difficult, particularly for the earliest members of the genus. There are several reasons for this. First, many fossil specimens dating to this time period are incomplete and poorly preserved, making them difficult to evaluate. Second, early Homo fossils appear quite variable in brain size, facial features, and teeth and body size, and there is not yet consensus about how to best make sense of this diversity. Finally, there is growing evidence that the evolution of the genus Homo proceeded in a mosaic pattern: in other words, these characteristics did not appear all at once in a single species; rather, they were patchily distributed in different species from different regions and time periods. Consequently, different researchers have come up with conflicting classification schemes depending on which criteria they think are most important.
In this chapter, we will take several pathways toward examining the origin and evolution of the genus Homo. First, we will explore the environmental conditions of the Pleistocene epoch in which the genus Homo evolved. Next we will examine the fossil evidence for the two principal species traditionally identified as early Homo: Homo habilis and Homo erectus. Then we will use data from fossils and archaeological sites to reconstruct the behavior of early members of Homo, including tool manufacture, subsistence practices, migratory patterns, and social structure. Finally, we will consider these together in an attempt to characterize the key adaptive strategies of early Homo and how they put our early ancestors on the trajectory that led to our own species, Homo sapiens.
CLIMATE CHANGE AND HUMAN EVOLUTION
A key goal in the study of human origins is to learn about the environmental pressures that may have shaped human evolution. As indicated in Chapter 7, scientists use a variety of techniques to reconstruct ancient environments. These include stable isotopes, core samples from oceans and lakes, windblown dust, analysis of geological formations and volcanoes, and fossils of ancient plant and animal communities. Such studies have provided valuable information about the environmental context of early Homo.
The early hominin species covered previously, such as Ardipithecus ramidus and Australopithecus afarensis, evolved during the late Pliocene epoch. The Pliocene (5.3 million to 2.6 million years ago) was marked by cooler and drier conditions, with ice caps forming permanently at the poles. Still, Earth’s climate during the Pliocene was considerably warmer and wetter than at present.
The subsequent epoch (2.6 million years to 11,000 years ago) ushered in major environmental change. The Pleistocene is popularly referred to as the Ice Age. Since the term “Ice Age” tends to conjure up images of glaciers and woolly mammoths, one would naturally assume that this was a period of uniformly cold climate around the globe. But this is not actually the case. Instead, climate became much more variable, cycling abruptly between warm/wet (interglacial) and cold/dry (glacial) cycles. The climate pattern was likely influenced by changes in Earth’s elliptical orbit around the sun. As is shown in Figure 10.2, each cycle averaged about 41,000 years during the early Pleistocene; the cycles then lengthened to about 100,000 years starting around 1.25 million years ago. Since mountain ranges, wind patterns, ocean currents, and volcanic activity can all influence climate pattern, climate change had extreme effects on the environment in some regions but less effects on others.
For a present-day example with which you might be familiar, consider the El Niño weather pattern. This is where warming of the Pacific Ocean in the equator region influences rainfall, hurricane frequency, and other weather activity in different parts of the world. During El Niño years, some areas get more rainfall than average and some get less. A recent El Niño in 2017 produced catastrophic flooding along the Peruvian coast, and one in 2015 led to drought and severe bushfires in Australia. If El Niños, despite being a predictable and well-known occurrence, can cause so much disruption to our technologically advanced society, imagine how vulnerable our ancestors must have been to climate change. An adaptive strategy that could buffer against this kind of uncertainty would have been extremely valuable.

Data on ancient geography and climate help us understand how our ancestors moved and migrated to different parts of the world, and the constraints under which they operated. When periods of global cooling dominated, sea levels were lower as more water was captured as glacial ice. This exposed continental margins and opened pathways between land masses. During glacial periods, the large Indonesian islands of Sumatra, Java, and Borneo were connected to the Southeast Asian mainland, while New Guinea was part of the southern landmass known as greater Australia. There was a land bridge connection between Britain and continental Europe, and an icy, treeless plain known as Beringia connected Northern Asia and Alaska. At the same time, glaciation made some northern areas inaccessible to human habitation. For example, there is evidence that hominin species were in Britain 950,000 years ago, but it does not appear that Britain was continuously occupied during this period. These early humans may have died out or been forced to abandon the region during glacial periods.
In Africa, paleoclimate research has determined that grasslands (shown in Figure 10.3) expanded and shrank multiple times during this period, even as they expanded over the long term (deMenocal 2014). From studies of fossils, paleontologists have been able to reconstruct Pleistocene animal communities and to consider how they were affected by the changing climate. Among the African animal populations, the number of grazing animal species such as antelope increased. Since our early ancestors were also part of this animal community, it is informative to consider how climate change caused changes in the home ranges and migration patterns of animals. Although the African and Eurasian continents are connected by land, the Sahara desert and the mountainous topography of North Africa serve as natural barriers to crossing. But the fossil record shows that animal species moved back and forth between Africa and Eurasia during the Pliocene and Pleistocene epochs. During the early Pleistocene, there is evidence of African mammal species such as baboons, hippos, antelope, and African buffalo migrating out of Africa into Eurasia during periods when drier conditions extended out from Africa into the Middle East (Belmaker 2010).

This changing environment was undoubtedly challenging for our ancestors, but it offered new opportunities for hominins to make a living. One solution adopted by some hominins was to specialize in feeding on the new types of plants growing in this landscape. As discussed in the previous chapter, the robust australopithecines probably developed their large molar teeth with thick enamel in order to exploit this particular dietary niche. Chemical analyses of robust australopith teeth show an isotopic signature of a diet where grasses and sedges are prominent, such as papyrus.
Members of the genus Homo took a different route. Faced with the unstable African climate and shifting landscape, they evolved bigger brains that enabled them to rely on cultural solutions such as crafting stone tools that opened up new foraging opportunities. This strategy of behavioral flexibility served them well during this unpredictable time and led to new innovations such as increased meat-eating, cooperative hunting, and the exploitation of new environments outside Africa.
HOMO HABILIS: THE EARLIEST MEMBERS OF OUR GENUS
Homo habilis has traditionally been considered the earliest species placed in the genus Homo. However, as we will see, there is substantial disagreement among paleoanthropologists about the fossils classified as Homo habilis, including whether they come from a single or multiple species, or even whether they should be part of the genus Homo at all.
Compared to the australopithecines in the previous chapter, Homo habilis has a somewhat larger brain size–an average of 650 cubic centimeters (cc) compared to less than 500 cc for Australopithecus. Additionally, the skull is more rounded and the face less prognathic. However, the postcranial remains show a body size and proportions similar to Australopithecus.
Known dates for fossils identified as Homo habilis range from about 2.5 million years ago to 1.7 million years ago. Recently, a partial lower jaw dated to 2.8 million years from the site of Ledi-Gararu in Ethiopia has been tentatively identified as belonging to the genus Homo (Villmoare et al. 2015). If this classification holds up, it would push the origins of our genus back even further.

Discovery and Naming
The first fossils to be named Homo habilis were discovered at the site of Olduvai Gorge in Tanzania, East Africa, by members of a team led by Louis and Mary Leakey (Fig. 10.4). The Leakey family had been conducting fieldwork in the area since the 1930s and had discovered other hominin fossils at the site, such as the robust Australopithecus boisei. The key specimen, a juvenile individual, was actually found by their 20-year-old son Jonathan Leakey. Louis Leakey invited South African paleoanthropologist Philip Tobias and British anatomist John Napier to reconstruct and analyze the remains. The fossil of the juvenile shown in Figure 10.5 (now known as OH-7) consisted of a lower jaw, parts of the parietal bones of the skull, and some hand and finger bones. Potassium-argon dating of the rock layers showed that the fossil dated to about 1.75 million years. In 1964, the team published their findings in the scientific journal Nature (Leakey et al. 1964). As described in the publication, the new fossils had smaller molar teeth that were less “bulgy” than australopithecine teeth. Although the primary specimen was not yet fully grown, an estimate of its anticipated adult brain size would make it somewhat larger-brained than australopithecines such as A. africanus. The hand bones were similar to humans’ in that they were capable of a precision grip. This increased the likelihood that stone tools found earlier at Olduvai Gorge were made by this group of hominins. Based on these findings, the authors inferred that it was a new species that should be classified in the genus Homo. They gave it the name Homo habilis, meaning “handy” or “skilled.”
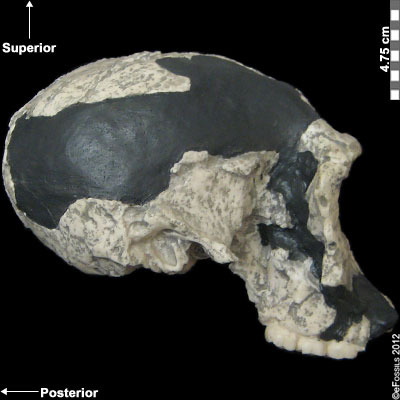
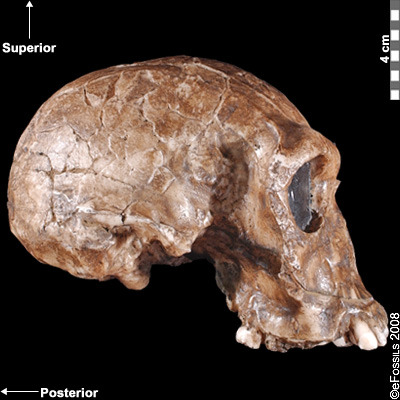
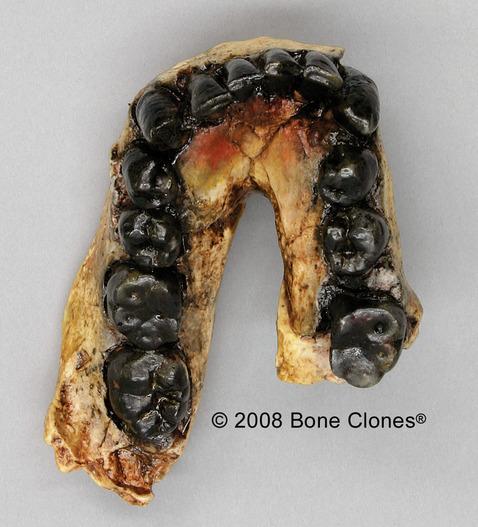
Figure 10.5 Homo habilis fossil specimens. From left to right they are: OH-24 (found at Olduvai Gorge), KNM-ER-1813 (from Koobi Fora, Kenya), and the jaw of OH-7, which was the type specimen found in 1960 at Olduvai Gorge, Tanzania.
|
Location of Fossils |
Dates |
Description |
|
Ledi-Gararu, Ethiopia |
2.8 mya |
Partial lower jaw with evidence of both Australopithecus and Homo traits; tentatively considered oldest Early Homo fossil evidence. |
|
Olduvai Gorge, Tanzania |
1.7 mya to 1.8 mya |
Several different specimens classified as Homo habilis, including the type specimen found by Leakey, a relatively complete foot, and a skull with a cranial capacity of about 600 cc. |
|
Koobi Fora, Lake Turkana Basin, Kenya |
1.9 mya |
Several fossils from the Lake Turkana basin show considerable size differences, leading some anthropologists to classify the larger specimen (KNM-ER-1470) as a separate species, Homo rudolfensis. |
|
Sterkfontein and other possible South African cave sites |
about 1.7 mya |
South African caves have yielded fragmentary remains identified as Homo habilis, but secure dates and specifics about the fossils are lacking. |
Figure 10.6 Key Homo habilis fossil locations and the corresponding fossils and dates.
Controversies over Classification of Homo habilis
How Many Species of Homo habilis?
Since this initial discovery, more fossils classified as Homo habilis were discovered in sites in East and South Africa in the 1970s and 1980s (Figure 10.6).. As more fossils joined the ranks of Homo habilis, several trends became apparent. First, the fossils were quite variable. While some resembled the fossil specimen first published by Leakey and colleagues, others had larger cranial capacity and tooth size. A well-preserved fossil skull from East Lake Turkana labeled KNM-ER-1470 displayed a larger cranial size along with a strikingly wide face reminiscent of a robust australopithecine. The diversity of the Homo habilis fossils prompted some scientists to question whether they displayed too much variation to all remain as part of the same species. They proposed splitting the fossils into at least two groups. The first group resembling the original small-brained specimen would retain the species name Homo habilis; the second group consisting of the larger-brained fossils such as KNM-ER-1470 would be assigned the new name of Homo rudolfensis (see Figure 10.7). Researchers who favored keeping all fossils in Homo habilis argued that sexual dimorphism, adaptation to local environments, or could be the cause of the differences. For example, modern human body size and body proportions are influenced by variations in climates and nutritional circumstances.
Given the incomplete and fragmentary fossil record from this time period, it is not surprising that classification has proved contentious. As a scholarly consensus has not yet emerged on the classification status of early Homo, this text will make use of the single (inclusive) Homo habilis species designation.
Homo habilis: Homo or Australopithecus?
There is also disagreement on whether Homo habilis legitimately belongs in the genus Homo. Most of the fossils first classified as Homo habilis consisted mainly of skulls and teeth. When arm, leg, and foot bones were later found, making it possible to estimate body size, they turned out to be quite small in stature with long arms and short legs. Analysis of the relative strength of limb bones suggested that the species, though bipedal, was much more adapted to arboreal climbing than Homo erectus and Homo sapiens (Ruff 2009). This has prompted some scientists to question whether Homo habilis behaved more like an australopithecine—with a shorter gait and the ability to move around in the trees (Wood and Collard 1999). They also questioned whether the brain size of Homo habilis was really that much larger than that of Australopithecus. They have proposed reclassifying some or all of the Homo habilis fossils into the genus Australopithecus, or even placing them into a newly created genus (Wood 2014).

Other scholars have interpreted the fossil evidence differently. A recent reanalysis of Homo habilis/rudolfensis fossils concluded that they sort into the genus Homo rather than Australopithecus (Figure 10.8). In particular, statistical analysis performed indicates that the Homo habilis fossils differ significantly in average cranial capacity from the australopithecines. They also note that some australopithecine species such as the recently discovered Australopithecus sediba have relatively long legs, so body size may not have been as significant as brain- and tooth-size differences (Anton et al. 2014).
|
Hominin |
Homo habilis |
|
Dates |
2.5 million years ago to 1.7 million years ago |
|
Region(s) |
East and South Africa |
|
Famous Discoveries |
Olduvai Gorge, Tanzania; Koobi Fora, Kenya; Sterkfontein, South Africa |
|
Brain Size |
650 cc average (range from 510 cc to 775 cc) |
|
Dentition |
Smaller teeth with thinner enamel compared to Australopithecus; parabolic dental arcade shape |
|
Cranial Features |
Rounder cranium and less facial prognathism than Australopithecus |
|
Postcranial Features |
Small stature; similar body plan to Australopithecus |
|
Culture |
Oldowan tools |
Figure 10.8 Summary features of Homo habilis.
HOMO HABILIS CULTURE AND LIFEWAYS
Early Stone Tools
The larger brains and smaller teeth of early Homo are linked to a different adaptive strategy than that of earlier hominins—one dependent on modifying rocks to make stone tools and exploit new food sources. Based on what we know from nonhuman-primate tool use, it is assumed that all hominins used tools of some sort. For example, australopithecines could have used digging sticks to extract the roots and tubers that were part of some species’ diets (though tools made from perishable material would leave no trace). As discussed in the previous chapter, stone tools almost certainly predated Homo habilis (possibly by Australopithecus garhi or the species responsible for the tools from Kenya dating to 3.7 million years ago). However, stone tools become more frequent at sites dating to about 2 million years ago, the time of Homo habilis (Roche, Blumenschine, and Shea 2009). This suggests that these hominins were increasingly reliant on stone tools to make a living.
Stone tools are assigned a good deal of importance in the study of human origins. Studying the form of the tools, the raw materials selected, and how they were made and used can provide insight into the thought processes of early humans and how they modified their environment in order to survive. Paleoanthropologists have traditionally classified collections of stone tools into industries, based on their form and mode of manufacture. There is not an exact correspondence between a tool industry and a hominin species; however, some general associations can be made between tool industries and particular hominins, locations, and time periods. The names for the four primary tool industries in human evolution (from oldest to most recent) are the Oldowan, Acheulean, Mousterian, and Upper Paleolithic.
The oldest stone tool industry is the , named after the site of Olduvai Gorge where the tools were first discovered. The time period of the Oldowan is generally considered to last from about 2.5 mya to 1.6 mya. The tools of this industry are described as “flake and chopper” tools—the choppers consisting of stone cobbles with a few flakes struck off them (Figure 10.9). To a casual observer, these tools might not look much different from randomly broken rocks. However, they are harder to make than their crude appearance suggests. The rock selected as the core must be struck by the rock serving as a hammerstone at just the right angle so that one or more flat flakes are removed. This requires selecting rocks that will fracture predictably instead of chunking, as well as the ability to plan ahead and envision the steps needed to create the finished product. The process leaves both the core and the flakes with sharp cutting edges that can be used for a variety of purposes.

Stone Tool Use and the Diet of Early Homo
What were the hominins doing with the tools? One key activity seems to have been butchering animals. Animal bones with cutmarks start appearing at sites with Oldowan tools. Studies of animal bones at the site show leg bones are often cracked open, suggesting that they were extracting the marrow from the bone cavities. It is interesting to consider whether the hominins hunted these animals or acquired them through other means. The butchered bones come from a variety of African mammals, ranging from small antelope to animals as big as wildebeest and elephants! It is difficult to envision slow, small-bodied Homo habilis with their Oldowan tools bringing down such large animals. One possibility is that the hominins were scavenging carcasses from lions and other large cats. Paleoanthropologist Robert Blumenschine has evaluated the scavenging hypothesis by directly observing the behavior of present-day animal carnivores and scavengers on the African savanna. From this, he inferred that there were scavenging opportunities for Plio-pleistocene hominins. When lions abandon a kill after eating their fill, scavenging animals arrive almost immediately to pick apart the carcass. By the time the slow-footed hominins arrived on the scene, the carcass would be mostly stripped of meat. However, if hominins could use stone tools to break into the leg bone cavities, they could get to the marrow, a fatty, calorie-dense source of protein (Blumenschine 1987).
Reconstructing activities that happened millions of years ago is obviously a difficult undertaking, and there is an active debate among anthropologists about whether scavenging or hunting was more commonly practiced during this time. Regardless of how they were acquiring the meat, all these activities suggest an important dietary shift from the way that the australopithecines were eating. The Oldowan toolmakers were exploiting a new ecological niche that provided them with more protein and calories. And it was not just limited to meat-eating—stone tool use could have made available numerous other subsistence opportunities. A study of microscopic wear patterns on a sample of Oldowan tools indicates that they were used for processing plant materials such as wood, roots or tubers, and grass seeds and stems (Lemorini et al. 2014). In fact, it has been pointed out that the Oldowan toolmakers’ cutting ability (whether for the purposes of consuming meat and plants or for making tools, shelters or clothing) represents a new and unique innovation, never seen before in the natural world! (Roche, Blumenschine, and Shea 2009).
Overall, increasing use of stone tools allowed hominins to expand their ecological niche and exert more control over their environment. As we’ll see shortly, this pattern continued and became more pronounced with Homo erectus.
HOMO ERECTUS: BIOLOGICAL AND CULTURAL INNOVATIONS
After 2 million years ago, a new hominin appeared on the scene. Known as Homo erectus, the prevailing scientific view was that this species was much more like us. These hominins were equipped with bigger brains and large bodies with limb proportions similar to our own. Perhaps most importantly, their way of life is now one that is recognizably human, with more advanced tools, hunting, use of fire, and colonizing new environments outside of Africa.
As will be apparent below, new data suggests that the story is not quite as simple. The fossil record for Homo erectus is much more abundant than that of Homo habilis, but it is also more complex and varied—both with regard to the fossils as well as the geographic context in which they are found. We will first summarize the anatomical characteristics that define Homo erectus, and then discuss the fossil evidence from Africa and the primary geographic regions outside Africa where the species has been located.
Homo erectus Anatomy
Compared to Homo habilis, Homo erectus showed increased brain size, smaller teeth, and a larger body. However, it also displayed key differences from later hominin species including our own.
Although the head of Homo erectus was less ape-like in appearance than the australopithecines, neither did it resemble modern humans (Figure 10.10). Compared to Homo habilis, Homo erectus had a larger brain size (average of about 900 cc compared to 650 cc to 750 cc). Instead of having a rounded shape like our skulls have, the erectus skull was long and low like a football, with a receding forehead, and a horizontal ridge called an that gave the back of the skull a squared-off appearance. The cranial bones are thicker than those of modern humans, and some Homo erectus skulls have a slight thickening along the sagittal suture called a . Large, shelf-like brow ridges hang over the eyes. The face shows less , and the back teeth are smaller than those of Homo habilis. Instead of a pointed chin, like ours, the mandible of Homo erectus recedes back.

Apart from these distinctive features, significant variation is present between Homo erectus fossils from different regions. Scientists have long noted differences between the fossils from Africa and those from Indonesia and China. For example, the Asian fossils tend to have a thicker skull and larger brow ridges than the African specimens, and the sagittal keel described above is more pronounced. Homo erectus fossils from the Republic of Georgia (described in the next section) also display distinctive characteristics. As with Homo habilis, this diversity has prompted a classification debate about whether or not Homo erectus should be split into multiple species. When African Homo erectus is characterized as a separate species, it is called Homo ergaster, while the Asian variant retains the erectus species name because it was discovered first. This text will use the species name Homo erectus for both variants.
Homo erectus was thought to have a body size and proportions more similar to modern humans. Unlike Homo habilis and the australopithecines, both of whom were small-statured with long arms and short legs, Homo erectus shows evidence of being fully committed to life on the ground. This meant long, powerfully muscled legs that enabled these hominins to cover more ground efficiently. Indeed, studies of the Homo erectus body form have linked several characteristics of the species to long-distance running in the more open savanna environment (Bramble and Lieberman 2004). Many experts think that hominins around this time had lost much of their body hair, were particularly efficient at sweating, and had darker-pigmented skin—all traits that would support the active lifestyle of such a large-bodied hominin (see Special Topic box).
Much of the information about the body form of Homo erectus comes from the Nariokotome fossil of the Homo erectus youth, described at the beginning of the chapter (see Figure 10.1). However, Homo erectus fossils are turning out to be more varied than previously thought. Homo erectus fossils from sites in Africa, as well as from Dmanisi, Georgia, show smaller body sizes than the Nariokotome boy’s. Even the Nariokotome skeleton itself has been reassessed to be quite a bit shorter (predicted to be closer to 5 feet 4 inches when fully grown, rather than over 6 feet), although there is still disagreement about which measurement is more accurate. One explanation for the range of body sizes could be adaptation to a range of different local environments, just as humans today show reduced body size in poor nutritional environments (Anton and Snodgrass 2012).
Homo erectus also shows some evidence of a reduction in sexual dimorphism in body size compared to the earlier australopithecines. In other words, Homo erectus males were only slightly larger in body size than females. The degree of sexual dimorphism among early hominin species is a contentious issue. It is a difficult characteristic to measure and assess in the fossil record, since fossils have to be complete enough to determine both body size and sex. However, if Homo erectus was less sexually dimorphic, it may signify changes in social organization within the species. If you recall from the chapter on primates, highly dimorphic species are those where males compete intensely for mating access to females. Decreased sexual dimorphism suggests that the lifestyle of Homo erectus may have been different from that of earlier hominins.
SPECIAL TOPICS: HOW WE BECAME HAIRLESS, SWEATY PRIMATES
As an anthropology instructor, one question about human evolution that students often ask me concerns human body hair—when did our ancestors lose it and why? It is assumed that our earliest ancestors were as hairy as modern-day apes. Today, though, we lack thick hair on most parts of our bodies except in the armpit and pubic regions and on the tops of our heads. Humans actually have about the same number of hair follicles per unit of skin as chimpanzees. But, the hairs on most of our body are so thin as to be practically invisible. When did we develop this peculiar pattern of hairlessness? Which selective pressures in our ancestral environment were responsible for this unusual characteristic?
Many experts believe that the driving force behind our loss of body hair was the need to effectively cool ourselves. Along with the lack of hair, humans are also distinguished by being exceptionally sweaty: we sweat larger quantities and more efficiently than any other primate. Humans have a larger amount of eccrine sweat glands than other primates and these glands generate an enormous volume of watery sweat. Sweating produces liquid on the skin that cools you off as it evaporates. It seems likely that hairlessness and sweating evolved together, as a recent DNA analysis has identified a shared genetic pathway between hair follicles and eccrine sweat gland production (Kamberov et al 2015).
Which particular environmental conditions led to such adaptations? In this chapter, we learned that the climate was a driving force behind many changes seen in the hominin lineage during the Pleistocene. At that time, the climate was increasingly arid and the forest canopy in parts of Africa was being replaced with a more open grassland environment, resulting in increased sun exposure for our ancestors. Compared to the earlier australopithecines, members of the genus Homo were also developing larger bodies and brains, starting to obtain meat by hunting or scavenging carcasses, and crafting sophisticated stone tools.
According to Nina Jablonski, an expert on the evolution of human skin, the loss of body hair and increased sweating capacity are part of the package of traits characterizing the genus Homo. While larger brains and long-legged bodies made it possible for humans to cover long distances while foraging, this new body form had to cool itself effectively to handle a more active lifestyle. Preventing the brain from overheating was especially critical. The ability to keep cool may have also enabled hominins to forage during the hottest part of the day, giving them an advantage over savanna predators, like lions, that typically rest during this time.
When did these changes occur? Although hair and soft tissue do not typically fossilize, there are several indirect methods that have been used to explore this question. One method tracks a human skin color gene. Since chimpanzees have light skin under their hair, it is probable that early hominins also had light skin color. Apes and other mammals with thick fur coats have protection against the sun’s rays. As our ancestors lost their fur, it is likely that increased melanin pigmentation was selected for to shield our ancestors from harmful ultraviolet radiation. A recent genetic analysis determined that one of the genes responsible for melanin production originated about 1.2 million years ago (Jablonski 2012).
Another line of evidence tracks the coevolution of a rather unpleasant human companion—the louse. A genetic study identified human body louse as the youngest of the three varieties of lice that infest humans, splitting off as a distinct variety around 70,000 years ago (Kittler, Kayser, and Stoneking 2003). Because human body lice can only spread through clothing, this may have been about the time when humans started to regularly wear clothing. However, the split between human head and pubic lice is estimated to have occurred much earlier, about three million years ago (Reed et al. 2007). When humans lost much of their body hair, lice that used to roam freely around the body were now confined to two areas: the head and pubic region. As a result of this “geographic” separation, the lice population split into two distinct groups.
Other explanations have also been suggested for the loss of human body hair. For example, being hairless has other advantages such as making it more difficult for skin parasites like lice, fleas, and ticks to live on us. Additionally, after bipedality evolved, hairless bodies would also make reproductive organs and female breasts more visible, suggesting that sexual selection may have played a role.
Homo erectus in Africa
Although the earliest discoveries of Homo erectus fossils were from Asia, the greatest quantity and best-preserved fossils of the species come from East African sites. The earliest fossils in Africa identified as Homo erectus come from the East African site of Koobi Fora, around Lake Turkana in Kenya, and are dated to about 1.8 million years ago. Other fossil remains have been found in East African sites in Kenya, Tanzania, and Ethiopia. Other notable African Homo erectus finds are a female pelvis from the site of Gona, Ethiopia (Simpson et al 2008), and a cranium from Olduvai Gorge known as Olduvai 9, thought to be about 1.4 million years old with massive brow ridges.
Homo erectus’ presence in South Africa is not well documented, though fossils thought to belong to the species have also been uncovered from the famed South African Swartkrans cave site along with stone tools and burned animal bones.
Regional Discoveries Outside Africa
It is generally agreed that Homo erectus was the first hominin to migrate out of Africa and colonize Asia and later Europe (although recent discoveries in Asia may challenge this view). Key locations and discoveries of Homo erectus fossils, along with the fossils’ estimated age are summarized below, and in Figure 10.12.

Figure 10.11 Map showing the locations of Homo erectus fossils around Africa and Eurasia.
Indonesia
The first discovery of Homo erectus was in the late 1800s in Java, Indonesia. A Dutch anatomist named Eugene Dubois searched for human fossils with the belief that since orangutans lived there, it might be a good place to look for remains of early humans. He discovered a portion of a skull, a femur, and some other bone fragments on a riverbank. While the femur looked human, the top of the skull was smaller and thicker than a modern person’s. Dubois named the fossil Pithecanthropus erectus (“upright ape-man”), popularized in the media at the time as “Java Man.” After later discoveries of similar fossils in China and Africa, they were combined into a single species (retaining the erectus name) under the genus Homo.
Homo erectus has a long history in Indonesia; further discoveries of fossils from Java were dated by argon dating to about 1.6 million to 1.8 million years. A cache of H. erectus fossils from the site of Ngandong in Java has yielded very recent dates of 43,000 years, although a more recent study with different dating methods concluded that they were much older—between 140,000 and 500,000 years old. Still, the possible existence of isolated, yet-to-be-discovered hominin populations in the region is of great interest to paleoanthropologists, especially given the discovery of the tiny Homo floresiensis fossils discovered on the nearby island of Flores, Indonesia, and the very recent announcement of possible tiny hominin fossils from the island of Luzon in the Philippines.
China
There is evidence of Homo erectus in China from several regions and time periods. Homo erectus fossils from northern China, collectively known as “Peking Man,” are some of the most famous human fossils in the world. Dated to about 400,000–700,000 years ago, they were excavated from the site of Zhoukoudian, near the outskirts of Beijing. Hundreds of bones and teeth, including six nearly complete skulls, were excavated from the cave in the 1920s and 1930s. Much of the fossils’ fame comes from the fact that they disappeared under mysterious circumstances. As Japan advanced into China during World War II, Chinese authorities, concerned for the security of the fossils, packed up the boxes and arranged for them to be transported to the United States. But in the chaos of the war, they vanished and were never heard about again. What exactly happened to them is murky—there are several conflicting accounts. Fortunately, an anatomist named Frans Weidenreich who had previously studied the bones had made casts and measurements of the skulls, so this valuable information was not lost. More recent excavations, at Longgushan “Dragon Bone Cave” at Zhoukoudian, of tools, living sites, and food remains, have revealed much about the lifestyle of Homo erectus during this time.
Despite this lengthy history of scientific research, China, compared to Africa, was perceived as somewhat peripheral to the study of hominin evolution. Although Homo erectus fossils have been found at several sites in China, with dates that make them comparable to those of Indonesian Homo erectus, none seemed to approximate the antiquity of African sites. The notable finds at sites like Nariokotome and Olorgesaille took center stage during the 1970s and 80’s, as scientists focused on elucidating the species’ anatomy and adaptations in its African homeland. In contrast, fewer research projects were focused on East Asian sites (Qiu 2016).
However, isolated claims of very ancient hominin occupation kept cropping up from different locations in Asia. While some were dismissed because of problems with dating methods or stratigraphic context, the 2018 publication of the discovery of stone tools from China dated to 2.1 million years caught everyone’s attention. Dated by paleomagnetic techniques that date the associated soils and windblown dust, these tools indicate that hominins in Asia predated those at Dmanisi by at least 300,000 years (Zhu et al. 2018). In fact, the tools are older than any Homo erectus fossils anywhere. Since no fossils were found with the tools, it isn’t known which species made them, but it opens up the intriguing possibility that hominins earlier than Homo erectus could have migrated out of Africa. These exciting new discoveries are shaking up previously held views of the East Asian human fossil record.
Western Eurasia
An extraordinary collection of fossils from the site of Dmanisi in the Republic of Georgia has revealed the presence of Homo erectus in Western Eurasia between 1.75 million and 1.86 million years ago. Dmanisi is located in the Caucasus mountains in Georgia. When archaeologists began excavating a medieval settlement near the town in the 1980s and came across the bones of extinct animals, they shifted their focus from the historic to the prehistoric era, but they probably did not anticipate going back quite so far in time! The first hominin fossils were discovered in the early 1990s, and since that time, at least five relatively well-preserved crania have been excavated.
There are several surprising things about the Dmanisi fossils. Compared to African Homo erectus, they have smaller brains and bodies. However, despite the small brain size, they show clear signs of Homo erectus traits such as heavy brow ridges and reduced facial prognathism. Paleoanthropologists have pointed to some aspects of their anatomy (such as the shoulders) that appear rather primitive, although their body proportions seem fully committed to terrestrial bipedalism. One explanation for these differences could be that the Dmanisi hominins represent a very early form of Homo erectus that left Africa before increases in brain and body size evolved in the African population.
Second, although the fossils at this location are from the same geological context, they show a great deal of variation in brain size and in facial features. One skull (Skull 5) has a cranial capacity of only 550 cc, smaller than many Homo habilis fossils, along with larger teeth and a protruding face. Scientists disagree on what these differences mean. Some contend that the Dmanisi fossils cannot all belong to a single species because each one is so different. Others assert that the variability of the Dmanisi fossils proves that they, along with all early Homo fossils, including H. habilis and H. rudolfensis, could all be grouped into Homo erectus (Lordikipanidze et al. 2013). Regardless of which point of view ends up dominating, the Dmanisi hominins are clearly central to the question of how to define the early members of the genus Homo.
Europe
Until recently, there was scant evidence of any Homo erectus presence in Europe, and it was assumed that hominins did not colonize Europe until much later than East Asia or Eurasia. One explanation for this was that the harsh ice age climate of Western Europe served as a barrier to living there. However, recent fossil finds from Spain suggest that Homo erectus could have made it into Europe over a million years ago. In 2008 a mandible from the Atapuerca region in Spain was discovered, dating to about 1.2 million years ago. A more extensive assemblage of fossils from the site of Gran Dolina in Atapuerca have been dated to about 800,000 years ago. In England in 2013 fossilized hominin footprints of adults and children dated to 950,000 years ago were found at the site of Happisburgh, Norfolk, which would make them the oldest human footprints found outside Africa (Ashton et al. 2014).
At this time, researchers aren’t in agreement as to whether the first Europeans belonged to Homo erectus proper or to a later descendent species. Some scientists refer to the early fossils from Spain by the species name, Homo antecessor.
|
Region |
Sites |
Dates |
Significance of Fossils |
|
East Africa |
East and West Lake Turkana, Kenya; Olduvai Gorge, Tanzania |
1.8 to 1.4 mya |
Earliest evidence of H. erectus; significant variation in skull and facial features. |
|
Western Eurasia |
Dmanisi, Republic of Georgia |
1.75 mya |
Smaller brains and bodies than H. erectus from other regions. |
|
Western Europe |
Atapuerca, Spain (Sima del Elefante and Gran Dolina caves) |
1.2 mya– 400,000 ya |
Partial jaw from Atapuerca is oldest evidence of H. erectus in Western Europe. Fossils from Gran Dolina (dated to about 800,000 years) sometimes referred to as H. antecessor. |
|
Indonesia |
Ngandong, Java; Sangiran, Java |
1.6 mya |
Early dispersal of H. erectus to East Asia; Asian H. erectus features. |
|
China |
Zhoukoudian, China; Loess Plateau (Lantian) |
780,000 – 400,000 ya 2.1 mya |
Large sample of H. erectus fossils and artifacts. Recent evidence of stone tools from Loess Plateau suggests great antiquity of Homo in East Asia. |
Figure 10.12 Regional comparisons of Homo erectus fossils.
HOMO ERECTUS LIFEWAYS
Now, our examination of Homo erectus will turn to its lifeways—how the species utilized its environment in order to survive. This includes making inferences about diet, technology, life history, environments occupied, and perhaps even social organization. As will be apparent, Homo erectus shows significant cultural innovations in these areas, some that you will probably recognize as more “human-like” than any of the hominins previously covered.
Tool Technology: Acheulean Tool Industry
In early African sites associated with Homo erectus, stone tools such as flakes and choppers identified to the Oldowan Industry dominate. Starting at about 1.5 million years ago, some Homo erectus populations began making different forms of tools. These tools–classified together as constituting the tool industry–are more complex in form and more consistent in their manufacture. Unlike the Oldowan tools, which were cobbles modified by striking off a few flakes, Acheulean toolmakers carefully shaped both sides of the tool. This type of technique, known as bifacial flaking, requires more planning and skill on the part of the toolmaker; he or she would need to be aware of principles of symmetry when crafting the tool. One of the most common tool forms, the handaxe, is shown in Figure 10.13. As with the tool illustrated below, handaxes tend to be thicker at the base and then come to a rounded point at the tip. Besides handaxes, forms such as scrapers, cleavers, and flake tools are present at Homo erectus sites.

One striking aspect of Acheulean tools is their uniformity. They are more standardized in form and mode of manufacture than the earlier Oldowan tools. For example, the aforementioned handaxes vary in size, but they are remarkably consistent in regard to their shape and proportions. They were also an incredibly stable tool form over time—lasting well over a million years with little change.
Curiously, the Acheulean tools so prominent at African sites are mostly absent in Homo erectus sites in East Asia. Instead, Oldowan-type choppers and scrapers are found at those sites. If this technology seemed to be so important to African Homo erectus, why didn’t East Asian Homo erectus also use the tools? One reason could be environmental differences between the two regions. Perhaps the rocks available in Asia weren’t of the material suitable for making the Acheulean handaxes. It has been suggested that Asian Homo erectus populations used perishable material such as bamboo to make tools. Another possibility is that Homo erectus (or even an earlier hominin) migrated to East Asia before the Acheulean technology developed in Africa. The recent discovery of the 2.1 million-year-old tools in China gives credence to this last explanation.
Tool Use and Cognitive Abilities of Homo erectus
What (if anything) do the Acheulean tools tell us about the mind of Homo erectus? Clearly, they took a fair amount of skill to manufacture. Apart from the actual shaping of the tool, other decisions made by toolmakers can reveal their use of foresight and planning. Did they just pick the most convenient rocks to make their tools, or did they search out a particular raw material that would be ideal for a particular tool? Analysis of Acheulean stone tools suggest that at some sites, the toolmakers selected their raw materials carefully—traveling to particular rock outcrops to quarry stones and perhaps even removing large slabs of rock at the quarries to get at the most desirable material. Such complex activities would require advanced planning. They also likely required cooperation and communication with other individuals, as such actions would be difficult to carry out solo. However, other Homo erectus sites lack evidence of such selectivity; instead of traveling even a short distance for better raw material, the hominins tended to use what was available in their immediate area (Shipton et al. 2018).
In contrast to Homo erectus tools, the tools of early modern Homo sapiens during the Upper Paleolithic display tremendous diversity across regions and time periods. Additionally, Upper Paleolithic tools and artifacts communicate information such as status and group membership. Such innovation and social signaling seem to have been absent in Homo erectus, suggesting that they had a different relationship with their tools than did Homo sapiens (Coolidge and Wynn 2017). Some scientists assert that these contrasts in tool form and manufacture may signify key cognitive differences between the species, such as the ability to use a complex language.
Subsistence and Diet
In reconstructing the diet of Homo erectus, researchers can draw from multiple lines of evidence. These include stone tools used by Homo erectus, animal bones and occasionally plant remains from Homo erectus sites, and the bones and teeth of the fossils themselves. These data sources suggest that compared to the australopithecines, Homo erectus consumed more animal protein. Coinciding with the appearance of Homo erectus fossils in Africa are archaeological sites with much more abundant stone tools and larger concentrations of butchered animal bones.
Meat Eating and Increased Brain Size
It makes sense that a larger body and brain would be correlated with a dietary shift to more calorically dense foods. This is because the brain is a very energetically greedy organ. Indeed, our own human brains require more than 20% of one’s calorie total intake to maintain. When biologists consider the evolution of intelligence in any animal species, it is often framed as a cost/benefit analysis: In order for large brains to evolve, there has to be a compelling benefit to having them and a way to generate enough energy to fuel them.
One solution that would allow for an increase in human brain size would be a corresponding reduction in the size of the digestive tract (gut). According to the “expensive tissue hypothesis,” initially formulated by Leslie Aiello and Peter Wheeler (1995), a smaller gut would allow for a larger brain without the need for a corresponding increase in the organism’s metabolic rate. Judging from their skeleton, australopithecines have a wider rib cage and trunk region more similar to apes than humans. It is thought that the australopithecines had large gut sizes similar to today’s great apes because they were eating mainly plant foods, which require more gut bacteria to digest. More meat in the diet would allow for a smaller gut and could also fuel the larger brain and body size seen in the genus Homo. Some researchers also believe that body fat percentages increased in hominins (particularly females) around this time, which would have allowed them to be better buffered against environmental disruption such as food shortages (Anton and Snodgrass 2012).
Evidence for Dietary Versatility in Homo erectus
As indicated above, evidence from archaeology and the inferences about Homo erectus body size suggest increased meat eating. How much hunting did Homo erectus engage in compared to the earlier Oldowan toolmakers? Although experts continue to debate the relative importance of hunting versus scavenging, there seems to be stronger evidence of hunting for these hominins. For example, at sites such as Olorgesailie in Kenya (Figure 10.14), there are numerous associations of Acheulean tools with butchered remains of large animals.

However, Homo erectus certainly ate more than just meat. Modern-day hunter-gatherer societies have been used as models for considering the behavior patterns and environmental constraints of our early ancestors. Plant foods make up the bulk of calories for most modern-day hunter-gatherer societies, since they are a much more reliable food source. It would make sense that we would see similar patterns among early hominins.
Studies of the tooth surfaces and microscopic wear patterns on hominin teeth indicate that Homo erectus ate a variety of foods, including some hard, brittle plant foods (Unger and Scott 2009). This would make sense, considering the environment was changing to be more dominated by grasslands in some areas. Roots, bulbs, and tubers (known as underground storage organs) of open savanna plants may have been a primary food source. Indeed, hunter-gatherer groups such as the Hadza of Tanzania rely heavily on such foods, especially during periods when game is scarce. In the unstable environment of the early Pleistocene, dietary versatility would be a definite advantage.
Tool Use, Cooking, and Fire
One key characteristic of the genus Homo is smaller teeth compared to Australopithecus. Why would teeth get smaller? Besides the change in the type of foods eaten, there may have also been changes in how food was prepared and consumed. Think about how you would eat if you didn’t have access to cutting tools. What you couldn’t rip apart with your hands would have to be bitten off with your teeth—actions that would require bigger, more powerful teeth and jaws. During this time, stone tools were becoming increasingly important. If hominins were using these tools to cut up, tenderize, and process meat and plants, they wouldn’t have to use their teeth so vigorously.
Cooking food could also have contributed to the reduction in tooth and jaw size. In fact, anthropologist Richard Wrangham (2009) asserts that cooking played a crucial role in human evolution. Cooking provides a head start in the digestive process because of how heat begins to break down food before food even enters the body, and it can help the body extract more nutrients out of meat and plant foods such as starchy tubers. According to Wrangham’s model, this improved diet had a number of far-reaching consequences for human evolution. Most importantly, it allowed for the larger brain and body size (and smaller gut size) seen in Homo erectus.
Obviously cooking requires fire, and the earliest use of fire is a fascinating topic in the study of human evolution. Fire, of course is not limited to humans; it occurs naturally as a result of lightning strikes. Like other wild animals, early hominins must have been terrified of wildfires, but at some point in time learned to control fire and put it to good use. Cooking, warmth, and scaring off wild animals are just some of the benefits of fire. Consider the potential social benefits of having a light source after dark. Rather than just going to sleep, members of the group could repair tools, plan the next day’s activities, or socialize—just as you might do sitting around a campfire with family or friends. Isn’t it intriguing to think about how such activities might have encouraged the development of language?
Documenting the earliest evidence of fire has been a contentious issue in archaeology because of the difficulty in distinguishing between human-controlled fire and natural burning at hominin sites. Burned areas and ash deposits must have direct associations with human activity to make a case for deliberate fire use. Examples might include the presence of wood ash in caves where trees don’t naturally grow, deep ash deposits in hearths lined with stones, or burned pieces of stone tools and butchered animal bones (Gao 2017). Unfortunately, such evidence is rare at ancient hominin sites, which have been profoundly altered by humans, animals, and geological forces over millions of years. Recently, newer methods—including microscopic analysis of burned rock and bone—have revealed clear evidence of fire use at Koobi Fora, Kenya, dating to 1.5 million years ago (Hlubik et al. 2017).
Migration out of Africa
Homo erectus is generally thought to be the first hominin species to leave the continent of Africa and settle in Eurasia in places such as the Republic of Georgia, Indonesia, and northern China. We previously discussed the timing and fossil evidence for the appearance of Homo erectus at those sites; now we can address why the species traveled such vast distances to these far-flung regions. To do this, we have to consider what we have learned about the biology, culture, and environmental circumstances of Homo erectus. The larger brain and body size of Homo erectus were fueled by a diet consisting of more meat, and longer more powerful legs made it possible to walk and run longer distances to acquire food. Since they were eating higher on the food chain, it was necessary for them to extend their home range to find sufficient game. Cultural developments including better stone tools and new technology such as fire gave them greater flexibility in adapting to different environments. Finally, the major Pleistocene climate shift discussed earlier in the chapter certainly played a role. Changes in air temperature, precipitation, access to water sources, and other habitat alteration had far-reaching effects on animal and plant communities; this included Homo erectus. If hominins were relying more on hunting, the migration patterns of their prey could have led them increasingly long distances.
Life History
The of a species refers to its overall pattern of growth, development, and reproduction during its lifetime, with the assumption being that these characteristics have been shaped by natural selection. Our species, Homo sapiens, is characterized by a unique life history pattern of slow development, a long period of juvenile dependence, and a long lifespan. Unlike the great apes whose offspring achieve early self-sufficiency, human children are dependent on their parents long after weaning. Additionally, human fathers and grandparents (particularly post-menopausal grandmothers) devote substantial time and energy to caring for their children.
who study modern hunter-gatherer societies have observed that foraging is no easy business (Figure 10.15). Members of these groups engage in complex foraging techniques that are difficult and take many years to master. An extended juvenile period gives children the time to acquire these skills. It also allows time for large human brains to grow and mature. On the back end, a longer developmental period results in skilled, successful adults, capable of living a long time (Hill and Kaplan 1999). Despite the time and energy demands, females could have offspring at more closely spaced intervals if they could depend on help from fathers and grandmothers (Hawkes et al. 1998).

What can the study of Homo erectus reveal about its life history pattern? Well-preserved fossils such as the Nariokotome boy can provide some insights. We know that apes such as chimpanzees reach maturity more quickly than humans, and there is some evidence that the australopithecines had a growth rate more akin to that of chimpanzees. Scientists have conducted extensive studies of the Nariokotome skeleton’s bones and teeth to assess growth and development. On the one hand, examination of the long bone ends (epiphyses) of the skeleton suggested that he was an early adolescent with a relatively large body mass, though growth had not yet been completed. On the other hand, study of the dentition, including measurement of microscopic layers of tooth enamel called , revealed a much younger age of 8 or 9. According to Christopher Dean and Holly Smith (2009), the best explanation for this discrepancy between the dental and skeletal age is that Homo erectus had its own distinct growth pattern—reaching maturity more slowly than chimpanzees but faster than Homo sapiens. This suggests that the human life history pattern of slow maturation and lengthy dependency was a more recent development. More work remains on refining this pattern for early Homo, but it is an important question, as it sheds light on how and when we developed our unique life history characteristics (Figure 10.16).
|
Hominin |
Homo erectus |
|
Dates |
1.8 million years ago to about 200,000 years ago |
|
Region(s) |
East and South Africa; West Eurasia; China and Southeast Asia |
|
Famous Discoveries |
Lake Turkana, Olorgesailie, Kenya; Zhoukoudian, China; Dmanisi, Republic of Georgia |
|
Brain Size |
Average 900 cc; range between 650 cc and 1,100 cc |
|
Dentition |
Smaller teeth than Homo habilis |
|
Cranial Features |
Long, low skull with robust features including thick cranial vault bones and large brow ridge, sagittal keel, and occipital torus |
|
Postcranial Features |
Larger body size compared to Homo habilis; body proportions (longer legs and shorter arms) similar to Homo sapiens |
|
Culture |
Acheulean tools (in Africa); evidence of increased hunting and meat-eating; use of fire; migration out of Africa |
Figure 10.16 Summary features of Homo erectus.
THE BIG PICTURE OF EARLY HOMO
We are discovering that the evolution of the genus Homo is more complex than what was previously thought. The earlier prevailing view of a simple progression from Australopithecus to Homo habilis to Homo erectus as clearly delineated stages in human evolution just doesn’t hold up anymore.
Variability in the Fossil Record of Early Homo
As is apparent from the information presented here, there is tremendous variability during this time. While fossils classified as Homo habilis show many of the characteristics of the genus Homo, such as brain expansion and smaller tooth size, the small body size and long arms are more akin to australopithecines. There is also tremendous variability within the fossils assigned to Homo habilis, so there is no consensus on whether it is a single or multiple species of Homo, a member of the genus Australopithecus, or even a yet-to-be-defined new genus. Similarly, there are considerable differences in skull morphology and body size and form of Homo erectus, of which some specimens show more similarity to Homo habilis than previously thought.
What does this diversity mean for how we should view early Homo? First, there isn’t an abrupt break between Australopithecus and Homo habilis or even between Homo habilis and Homo erectus. Characteristics we define as Homo don’t appear as a unified package; they appear in the fossil record at different times. This is known as . Indeed, fossil finds such as Australopithecus sediba, and Homo naledi and Homo floresiensis discussed in the next chapter, have displayed unexpected combinations of primitive and derived traits.
We can consider several explanations for the diversity we see within early Homo from about 2.5 million to 1.5 million years ago. One possibility is the existence of multiple contemporaneous species of early Homo during this period. In light of the pattern of environmental instability discussed earlier, it shouldn’t be surprising to see fossils from different parts of Africa and Eurasia display tremendous variability. Multiple hominin forms could also evolve in the same region, as they diversified in order to occupy different ecological niches. However, even the presence of multiple species of hominin does not preclude their interacting and interbreeding with one another. As you’ll see in the next chapter, sequencing of ancient hominin genomes has led to deeper understanding of genetic relationships between extinct species such as the Neanderthals and Denisovans.
Diversity of brain and body sizes could also reflect developmental plasticity—short-term adaptations within a lifetime (Anton, Potts, and Aiello 2014). These have the advantage of being more flexible than genetic natural selection, which could only occur over many generations. For example, among human populations today, different body sizes are thought to be adaptations to different climate or nutritional environments. Keeping in mind that the climate was intensely variable, wouldn’t a more flexible strategy of adaptation be valuable under these conditions?
Trends in the Behavior of Early Homo
New discoveries are also questioning old assumptions about the behavior of Homo habilis and Homo erectus. Just as the fossil evidence doesn’t neatly separate Australopithecus and Homo, evidence of the lifeways of early Homo show similar diversity. For example, one of the traditional dividing lines between Homo and Australopithecus was thought to be stone tools: Homo made them; Australopithecus didn’t. However, the recent discovery of stone tools from Kenya dating to 3.3 million years ago challenges this point of view. Similarly, the belief that Homo erectus was the first species to settle outside Africa may now come into question with the report of 2.1 million-year-old stone tools from China. If this find is supported by additional evidence, it may cause a reevaluation of Homo erectus being first to leave. Instead, there could have been multiple earlier migrations of hominins such as Homo habilis or even Australopithecus species.
These various lines of evidence about the genus Homo point out the need for a more nuanced view of this period of human evolution. Rather than obvious demarcations between species and their corresponding behavioral advancements, it now looks like many behaviors were shared among species. Earlier hominins that we previously didn’t think had the capability could have been doing things like expanding out of Africa or using stone tools. Meanwhile, some other hominins that we had considered more advanced didn’t actually have the full suite of “human” characteristics previously expected.
From a student’s perspective, all this complexity probably seems frustrating. It would be ideal if the human story were a straightforward, sequential narrative. Unfortunately, it seems that human evolution was not a nice, neat trajectory of increasingly humanlike traits and behaviors; rather, it is emblematic of the untidy but exciting nature of the study of human evolution.
Despite the haziness dominating the early Homo narrative, we can identify some overall trends for the million-year period associated with early Homo. These trends include brain expansion, a reduction in facial prognathism, smaller jaw and tooth size, larger body size, and evidence of full terrestrial bipedalism. These traits are associated with a key behavioral shift that emphasizes culture as a flexible strategy to adapt to unpredictable environmental circumstances. Included in this repertoire are the creation and use of stone tools to process meat obtained by scavenging and later hunting, a utilization of fire and cooking, and the roots of the human life history pattern of prolonged childhood, cooperation in child raising, and the practice of skilled foraging techniques. In fact, it’s apparent that the cultural innovations are driving the biological changes, and vice versa, fueling a feedback loop that will continue during the later stages of human evolution.
Review Questions
- Describe the climate during the early Pleistocene. Explain why climate is important for understanding the evolution of early Homo.
- List the key anatomical characteristics that are generally agreed to define the genus Homo.
- Why has classification of early Homo fossils proved difficult? What are some explanations for the variability seen in these fossils?
- Compare and contrast the Oldowan and Acheulean tool industries.
- Name some specific behaviors associated with Homo erectus in the areas of tool use, subsistence practices, migration patterns, and other cultural innovations.
Key Terms
Acheulean: Tool industry characterized by teardrop-shaped stone handaxes flaked on both sides.
Developmental plasticity: The capability of an organism to modify its phenotype during development in response to environmental cues.
Human behavioral ecology: Study of human behavior from an evolutionary and ecological perspective.
Life history: The broad pattern of a species’ life cycle including development, reproduction, and longevity.
Mosaic evolution: Different characteristics evolve at different rates and appear at different stages.
Occipital torus: A ridge on the occipital bone in the back of the skull.
Oldowan: Earliest stone-tool industry consisting of simple flakes and choppers.
Perikymata: Microscopic ridges on the surface of tooth enamel that serve as markers of tooth development.
Pleistocene: Geological epoch dating from 2.6 million years ago to about 11,000 years ago.
Prognathism: Condition where the lower face and jaw protrude forward from a vertical plane.
Sagittal keel: A thickened area along the top of the skull.
About the Author
Bonnie Yoshida-Levine, Ph.D.
Grossmont College, bonnie.yoshida@gcccd.edu

Bonnie Yoshida-Levine is an instructor of anthropology at Grossmont College, where she teaches biological anthropology and archaeology. She received her bachelor’s degree in history from the University of California, Los Angeles, and her M.A. and Ph.D. degrees in anthropology from the University of California, Santa Barbara. Her dissertation research focused on the bioarchaeology of early civilizations in north coastal Peru. Bonnie has also collaborated on archaeological field projects in Bolivia and coastal California.
For Further Exploration
Boaz, Noel Thomas, and Russell L. Ciochon. 2004. Dragon Bone Hill: An Ice-Age Saga of Homo erectus. New York: Oxford University Press.
Human Evolution by the Smithsonian Institution: http://humanorigins.si.edu/ Produced by the Smithsonian National Museum of Natural History, this website covers many aspects of human evolution including 3-D models of hominin fossils.
Jablonski, Nina G. 2010. “The Naked Truth.” Scientific American 302 (2): 42–49.
Lewin, Roger, and Robert A. Foley. 2004. Principles of Human Evolution. Oxford, UK: Blackwell Publishing.
Stoneking, Mark. 2015. “Of Lice and Men: The Molecular Evolution of Human Lice.” Lecture, Center for Academic Research & Training in Anthropogeny, San Diego, California, October 16, 2015. https://carta.anthropogeny.org/events/unique-features-human-skin
Tarlach, Gemma. 2015. “The First Humans to Know Winter.” Discover, February 26.
Ungar, Peter S. 2017. Evolution’s Bite : A Story of Teeth, Diet, and Human Origins. Princeton, NJ: Princeton University Press.
Wrangham, Richard. 2009. Catching Fire: How Cooking Made Us Human. New York: Basic Books.
References
Aiello, Leslie C., and Peter Wheeler. 1995. “The Expensive-Tissue Hypothesis.” Current Anthropology 36 (2): 199–221.
Anton, Susan C., Richard Potts, and Leslie C. Aiello. 2014. “Evolution of Early Homo: An Integrated Biological Perspective.” Science 345 (6192) doi: 10.1126/science.1236828
Anton, Susan C., and J. Josh Snodgrass. 2012. “Origins and Evolution of Genus Homo: New Perspectives.” Current Anthropology 53 (S6): S479–S496.
Ashton, Nick, Simon G. Lewis, Isabelle De Groote, Sarah M. Duffy, Martin Bates, Richard Bates, Peter Hoare, Mark Lewis, Simon A. Parfitt, Sylvia Peglar, Craig Williams, and Chris Stringer. 2014. “Hominin Footprints from Early Pleistocene Deposits at Happisburgh, UK.” PLOS ONE 9 (2): e88329.
Belmaker, Miriam. 2010. “Early Pleistocene Faunal Connections between Africa and Eurasia: An Ecological Perspective.” In Out of Africa I: The First Hominin Colonization of Eurasia, edited by John G. Fleagle, John J. Shea, Frederick E. Grine, Andrea L. Baden, and Richard E. Leakey, 183–205. Dordrecht: Springer Netherlands.
Blumenschine, Robert, Henry T. Bunn, Valerius Geist, Fumiko Ikawa-Smith, Curtis W. Marean, Anthony G. Payne, John Tooby, J. Nikolaas, and Van Der Merwe. 1987. “Characteristics of an Early Hominid Scavenging Niche [and Comments and Reply].” Current Anthropology 28 (4): 383–407.
Bower, Bruce. 2004. “Evolution’s Buggy Ride.” Science News 166 (15): 230–230.
Bramble, Dennis M., and Daniel E. Lieberman. 2004. “Endurance Running and the Evolution of Homo.” Nature 432: 345–352.
Coolidge, Frederick L., and Thomas Grant Wynn. 2017. The Rise of Homo Sapiens: The Evolution of Modern Thinking. New York: Oxford University Press.
Dean, M. Christopher, and B. Holly Smith. 2009. “Growth and Development of the Nariokotome Youth, KNM-WT 15000.” In The First Humans–Origin and Early Evolution of the Genus Homo: Contributions from the Third Stony Brook Human Evolution Symposium and Workshop October 3–7, 2006, edited by Frederick E. Grine, John G. Fleagle, and Richard E. Leakey, 101–120. Dordrecht: Springer Netherlands.
deMenocal, Peter B. 2014. “Climate Shocks.” Scientific American 311 (3): 48–53.
Dennell, Robin, and Wil Roebroeks. 2005. “An Asian Perspective on Early Human Dispersal from Africa.” Nature 438 (7071): 1099–1104.
Gao, Xing, Shuangquan Zhang, Yue Zhang, and Fuyou Chen. 2017. “Evidence of Hominin Use and Maintenance of Fire at Zhoukoudian.” Current Anthropology 58 (Number S16): S267–S277.
Hawkes, Kristen, James F. O’Connell, Nicholas G. Blurton Jones, Helen Alvarez, and Eric L. Charnov. 1998. “Grandmothering, Menopause, and the Evolution of Human Life Histories.” Proceedings of the National Academy of Sciences 95 (3): 1336–1339.
Hill, Kim, and Hillard Kaplan. 1999. “Life History Traits in Humans: Theory and Empirical Studies.” Annual Review of Anthropology 28 (1): 397–430.
Hlubik, Sarah, Francesco Berna, Craig Feibel, David Braun, and John W. K. Harris. 2017. “Researching the Nature of Fire at 1.5 Mya on the Site of FxJj20 AB, Koobi Fora, Kenya, Using High-Resolution Spatial Analysis and FTIR Spectrometry.” Current Anthropology 58 (S16): S243–S257.
Jablonski, Nina G. 2010. “The Naked Truth.” Scientific American 302 (2): 42–49.
Kamberov, Yana G., Elinor K. Karlsson, Gerda L. Kamberova, Daniel E. Lieberman, Pardis C. Sabeti, Bruce A. Morgan, and Clifford J. Tabin. 2015. “A Genetic Basis of Variation in Eccrine Sweat Gland and Hair Follicle Density.” Proceedings of the National Academy of Sciences 112 (32): 9932–9937.
Kittler, R., M. Kayser, and M. Stoneking. 2003. “Molecular Evolution of Pediculus Humanus and the Origin of Clothing.” Current Biology 13 (16): 1414-7.
Leakey, Louis S. B., Phillip V. Tobias, and John R. Napier. 1964. “A New Species of Genus Homo from Olduvai Gorge.” Nature 202: 308–312.
Lemorini, Cristina, Thomas W. Plummer, David R. Braun, Alyssa N. Crittenden, Peter W. Ditchfield, Laura C. Bishop, Fritz Hertel, James S. Oliver, Frank W. Marlowe, Margaret J. Schoeninger, and Richard Potts. 2014. “Old Stones’ Song: Use-wear Experiments and Analysis of the Oldowan Quartz and Quartzite Assemblage from Kanjera South (Kenya).” Journal of Human Evolution 72: 10–25.
Lordkipanidze, David, Marcia S. Ponce de León, Ann Margvelashvili, Yoel Rak, G. Philip Rightmire, Abesalom Vekua, and Christoph P. E. Zollikofer. 2013. “A Complete Skull from Dmanisi, Georgia, and the Evolutionary Biology of Early Homo.” Science 342 (6156): 326–333.
Qiu, Jane. 2016. “How China Is Rewriting the Book on Human Origins.” Nature 535: 22-25.
Reed, David L., Jessica E. Light, Julie M. Allen, and Jeremy J. Kirchman. 2007. “Pair of Lice Lost or Parasites Regained: The Evolutionary History of Anthropoid Primate Lice.” BMC Biology 5 (1):7. doi: 10.1186/1741-7007-5-7.
Roche, Helene, Robert J. Blumenschine, and John J. Shea. 2009. “Origins and Adaptations of Early Homo: What Archeology Tells Us.” In The First Humans: Origin and Early Evolution of the Genus Homo, edited by Frederick E. Grine, John G. Fleagle, and Richard E. Leakey, 135-147. New York: Springer.
Rogers, Alan R., David Iltis, and Stephen Wooding. 2004. “Genetic Variation at the MC1R l Locus and the Time since Loss of Human Body Hair.” Current Anthropology 45 (1): 105-108.
Ruff, Christopher. 2009. “Relative Limb Strength and Locomotion in Homo habilis.” American Journal of Physical Anthropology 138 (1): 90–100.
Shipton, Ceri, James Blinkhorn, Paul S. Breeze, Patrick Cuthbertson, Nick Drake, Huw S. Groucutt, Richard P. Jennings, Ash Parton, Eleanor M. L. Scerri, Abdullah Alsharekh, and Michael D. Petraglia. 2018. “Acheulean Technology and Landscape Use at Dawadmi, Central Arabia.” PloS one 13 (7): e0200497–e0200497.
Simpson, Scott W., Jay Quade, Naomi E. Levin, Robert Butler, Guillaume Dupont-Nivet, Melanie Everett, and Sileshi Semaw. 2008. “A Female Homo erectus Pelvis from Gona, Ethiopia.” Science 322 (5904): 1089–1092.
Ungar, Peter S and Robert S. Scott. 2009. “Dental Evidence for Diets of Early Homo.” In The First Humans: Origin and Early Evolution of the Genus Homo, edited by Frederick E. Grine, John G. Fleagle, and Richard E. Leakey, 121-134. New York: Springer.
Villmoare, Brian, William H. Kimbel, Chalachew Seyoum, Christopher J. Campisano, Erin N. DiMaggio, John Rowan, David R. Braun, J. Ramón Arrowsmith, and Kaye E. Reed. 2015. “Early Homo at 2.8 Ma From Ledi-Geraru, Afar, Ethiopia.” Science 347 (6228): 1352–1355.
Wood, Bernard. 2014. “Human Evolution: Fifty Years after Homo habilis.” Nature 508 (7494): 31–33.
Wood, Bernard, and Mark Collard. 1999. “The Changing Face of Genus Homo.” Evolutionary Anthropology 8 (6): 195–207.
Wrangham, Richard. 2009. Catching Fire: How Cooking Made Us Human. New York: Basic Books.
Zhu, Zhaoyu, Robin Dennell, Weiwen Huang, Yi Wu, Shifan Qiu, Shixia Yang, and Zhiguo Rao. 2018. “Hominin Occupation of the Chinese Loess Plateau Since about 2.1 Million Years Ago.” Nature 559: 608–612.
Acknowledgments
The author gratefully acknowledges funding from the California Community Colleges Chancellor’s Office Zero Textbook Cost Degree Grant Program—Implementation Phase 2.
Figure Attributions
Figure 10.1a KNM-WT 15000 Turkana Boy Skeleton by Smithsonian [exhibit: Human Evolution Evidence, Human Fossils, Fossils, KNM-WT 15000] is copyrighted and used for educational and non-commercial purposes as outlined by the Smithsonian.
Figure 10.1b MNP – Turkanajunge 2 by Wolfgang Sauber (photograph) and E. Daynes (sculpture) is used under a CC BY-SA 4.0 License.
Figure 10.2 Five Myr Climate Change by Dragons flight (Robert A. Rohde), based on data from Lisiechi and Raymo (2005), is used under a CC BY-SA 3.0 License.
Figure 10.3 Savanna grasslands of East Africa by International Livestock Research Institute (ILRI)/Elsworth is used under a CC BY-NC-SA 2.0 License.
Figure 10.4 Homo habilis site map original to Explorations: An Open Invitation to Biological Anthropology by Chelsea Barron at GeoPlace, California State University, Chico is under a CC BY-NC 4.0 License.
Figure 10.5a Homo habilis: OH 24 lateral right view by eFossils is copyrighted and used for non-commercial purposes as outlined by eFossils.
Figure 10.5b Homo habilis: KNM-ER 1813 lateral right view by eFossils is copyrighted and used for non-commercial purposes as outlined by eFossils.
Figure 10.5c Homo habilis OH 7 Jaw by ©BoneClones is used by permission and available here under a CC BY-NC 4.0 License.
Figure 10.6 Homo habilis table original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
Figure 10.7 Homo rudolfensis Cranium KNM-ER 1470 by ©BoneClones is used by permission and available here under a CC BY-NC 4.0 License.
Figure 10.8 Summary features of Homo habilis original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
Figure 10.9 Chopping tool by José-Manuel Benito Álvarez is used under a CC BY-SA 2.5 License.
Figure 10.10 Homo erectus: Sangiran 17 lateral left view by eFossils is copyrighted and used for non-commercial purposes as outlined by eFossils.
Figure 10.11 Homo erectus site map original to Explorations: An Open Invitation to Biological Anthropology by Chelsea Barron at GeoPlace, California State University, Chico is under a CC BY-NC 4.0 License.
Figure 10.12 Regional comparisons of Homo erectus fossils original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
Figure 10.13 Hand axe spanish by Locutus Borg has been designated to the public domain (CC0).
Figure 10.14 Elephant Butchery Site Olorgesailie, Kenya by Smithsonian [exhibit: Human Evolution Research, East African Research Projects, Olorgesailie, Kenya] is copyrighted and used for educational and non-commercial purposes as outlined by the Smithsonian.
Figure 10.15 Hadzabe1 by Idobi is used under a CC BY-SA 3.0 License.
Figure 10.16 Summary features of Homo erectus original to Explorations: An Open Invitation to Biological Anthropology is under a CC BY-NC 4.0 License.
Geological epoch dating from 2.6 million years ago to about 11,000 years ago.
The capability of an organism to modify its phenotype during development in response to environmental cues.
Earliest stone-tool industry consisting of simple flakes and choppers.
A ridge on the occipital bone in the back of the skull.
A thickened area along the top of the skull.
Condition where the lower face and jaw protrude forward from a vertical plane.
Tool industry characterized by teardrop-shaped stone handaxes flaked on both sides.
Refers to an organism’s pace of growth, reproduction, life span, etc.
The field of anthropology that explores how ecological factors and evolutionary history combine to influence how humans behave.
Microscopic ridges on the surface of tooth enamel that serve as markers of tooth development.
The concept that evolutionary change does not occur homogeneously throughout the body in organisms.
