Anatomy and Physiology
18 Blood

Kuʻu ēwe, kuʻu piko, kuʻu iwi, kuʻu koko.
My umbilical cord, my navel, my bones, my blood.
Said of a very close relative.
ʻŌlelo Noʻeau, compiled by Mary Kawena Pukui, #1932
Introduction

Figure 18.1: Blood Cells: Colored Scanning electron microscope (SEM)-picture: , thrombocyte, (from left to right)
![]() Chapter Learning Outcomes
Chapter Learning Outcomes
- Identify the primary functions of blood, its fluid, and cellular components, and its physical characteristics
- Identify the most important proteins and other solutes present in blood plasma
- Describe the formation of the formed element components of blood
- Discuss the structure and function of red blood cells and hemoglobin
- Identify the various ways an individual can increase red blood cells including blood doping
- Classify and characterize white blood cells
- Describe the structure of platelets and explain the process of hemostasis
- Explain the significance of AB and s in blood transfusions
- Discuss a variety of blood disorders
Have you thought about the importance of ? Blood is vital to life. So vital that there are many homeostatic mechanisms put into place to stop losing blood (in the case of bleeding) or regulating the amount of dissolved and nondissolved substances in the blood (such as pH). This amazing fluid acts as a delivery medium for vital components essential for life. It circulates within the blood vessels transporting gasses, nutrients, and hormones to the cells while removing wastes. Blood contains formed elements, such as erythrocytes, leukocytes, and , in a fluid matrix known as . As you will see in this chapter, this fluid that suspends the formed elements is composed mainly of water but also contains various essential cells and proteins.
18.1 Functions and Properties of Blood
![]() 18.1 Learning Outcomes
18.1 Learning Outcomes
- Identify the primary functions of blood in transportation, defense, and maintenance of homeostasis
- Describe the characteristic of blood
- Name the fluid component of blood and the three major types of formed elements, and identify their relative proportions in a blood sample
- Discuss the unique physical characteristics of blood
- Identify the composition of blood plasma, including its most important solutes and plasma proteins
The primary functions of the blood are transportation, regulation, and defense.
Transportation
Blood serves as the highway system throughout the body. It transports formed elements and dissolved substances. For instance, blood transports oxygen from the lungs and carbon dioxide to the lungs for gas exchange. Blood absorbs nutrients from the gastrointestinal tract and moves waste from the tissues. Hormones are released into the bloodstream by endocrine glands and carried throughout the entire cardiovascular system.
![]() Local Issue
Local Issue
Blood and Homeostasis
Blood is involved in regulating the pH of the fluids and tissues of our body. Chemical buffers, such as bicarbonate, bind and release hydrogen ions to keep blood pH within homeostatic levels. Blood regulates the body’s temperature through the absorption of heat from body cells as the blood moves through the blood vessels of the tissues of the body. As blood moves through the blood vessels of the skin, there is a release of heat from the blood to the body’s surface. Blood also helps balance the distribution of fluids in the body. For instance, fluid components within the blood plasma in the capillaries move back and forth with the fluid found within the interstitial fluid around the cells.
Blood and Immunity
The blood carries different types of leukocytes that will be sent to defend the body against foreign pathogens and diseases. In addition, the platelets within the blood help prevent excessive blood loss in cases of bleeding.
Blood Characteristics
The physical characteristics of the blood are color, volume, viscosity, plasma concentration, blood pH, and temperature.
- Color: The color of the blood will depend on whether the blood is rich or poor in oxygen. Oxygen-poor blood is dark red and not blue, as one may think. In comparison, oxygen-rich blood is bright red. As each oxygen molecule binds to , there is a slight change in hemoglobin structure. This causes it to reflect different wavelengths of light as it loads with oxygen. The wavelengths of light are reflected to our retinas and affect what color our eyes see. For example, our superficial veins just under the skin appear as blue, but that is because the skin absorbs the red wavelength of light leaving blue to be reflected to the eye. Thus, our visual perception of the veins is blue and blue only.
- Volume: Blood volume is primarily based on body mass and to a lesser extent body composition. Simply put, the larger the person the more blood volume. For example, an adult weighing 150 lbs (68 kg) will have approximately 5 liters of blood, whereas a 250 lb (113 kg) person will have approximately 8.5 liters of blood.
- Plasma concentration: The relative concentration of solutes is known as plasma concentration, and this concentration determines the movement of fluids between the plasma and the interstitial fluid by osmosis.
- Blood pH: Blood pH is slightly alkaline and between 7.35 and 7.45. It is tightly regulated to remain in this homeostatic “sweet spot”. If the blood pH fluctuates significantly, there can be devastating changes that can lead to disease and death. Recall that changes in pH may cause denaturing of proteins. This denaturing leads to a change in the shape of the blood proteins as their formation depends on the concentration of hydrogen ions. If the proteins change their shape, it also affects their functions.
Blood Components
Remember when you had blood drawn from a vein in your arm for lab tests? One of the tests is called , which measures the percentage of erythrocytes (red blood cells) in the blood. Altogether, the blood plasma and the formed elements are called . This whole blood can be separated using a piece of lab equipment called a centrifuge. This equipment spins the blood within a small tube and separates the different components of blood-based on their density. From the bottom, where the heavier components are, to the top, the following are the components:
- Erythrocytes are located at the bottom, forming about 44% of a blood sample
- Leukocytes and platelets form the middle layer. This very thin layer is called the and makes up about 1% of the blood sample.
- Plasma is the liquid localized at the top of the tube and makes up around 55% of the blood sample.
Regarding the values for the hematocrit, males show a higher percentage (42%-52%), while females (37%-47%) show a lower rate. Please see the Figure below to compare these values. Males have a higher percentage of these “formed elements” as they produce more androgens. Red blood cell production responds positively to androgen secretion. since they have more of the hormone testosterone. This hormone stimulates the production of another hormone, called , from the kidneys. EPO triggers the production of erythrocytes. In the Figure below (link to Figure Composition of Blood), you can also see that a lower hematocrit indicates that a person has . In comparison, a higher hematocrit suggests that a person has that could be caused by dehydration or blood doping.

Figure 18.2 Composition of Blood https://openstax.org/books/anatomy-and-physiology/pages/18-1-an-overview-of-blood#fig-ch19_01_01
![]() Deep dive: Hematocrit levels
Deep dive: Hematocrit levels
Blood Plasma Composition
Blood plasma is composed of mostly water (about 90%), and the rest of the plasma consists of proteins and other solutes, such as nutrients (glucose), electrolytes (sodium ions), gases (oxygen and carbon dioxide), and wastes (urea).
Plasma and plasma proteins
| Blood protein | Normal level | % | Function |
|---|---|---|---|
| Albumins | 3.5-5.0 g/dl | 55% | create and maintain osmotic pressure; transport insoluble molecules |
| Globulins | 2.0-2.5 g/dl | 38% | participate in immune system |
| Fibrinogen | 0.2-0.45 g/dl | 7% | Blood coagulation |
| Regulatory proteins | <1% | Regulation of gene expression | |
| Clotting factors | <1% | Conversion of fibrinogen into fibrin |

Figure 18.3: Blood Plasma: A chart and graph showing the breakdown and numbers of blood plasma including the breakdown of proteins and other components.

Table 18.1: Major Blood Components
Extracellular fluid is the fluid found outside of the cells. Plasma is categorized as extracellular fluid. Plasma has a higher concentration of proteins compared to the interstitial fluid (the fluid between cells). Both fluids share a similar composition in electrolytes, nutrients, and wastes.
Due to the concentration of plasma proteins and solutes dissolved within the plasma, blood is classified as a colloid where you cannot see through the solution and it appears to be opaque. The exerted by the plasma proteins helps to pull fluids into the blood at the capillary level. This prevents excess fluid from leaving the blood and entering the interstitial fluid. This osmotic pressure helps keep blood volume and blood pressure at homeostatic levels.
The three major categories of plasma proteins are as follows:
is produced and released by the liver. It is the most abundant of plasma proteins. Albumin usually accounts for approximately 54-60 percent of the total plasma protein content in whole blood.
Albumin molecules serve as binding proteins and transport vehicles for fatty acids and steroid hormones. Recall that lipids are hydrophobic and will not simply dissolve in the watery portion of blood; however, their binding to albumin enables their transport in the plasma composed chiefly of water. It might be helpful to think of plasma proteins as barges in the sea of blood. Like barges delivering containers across the seas, plasma proteins can bind and transport hydrophobic substances across the bloodstream. Albumin, as it represents the majority of plasma proteins is also the most significant contributor to the osmotic pressure of blood; its presence holds water inside the blood vessels and draws water from the tissues, across capillary walls, and into the bloodstream. This, in turn, helps to maintain both blood volume and blood pressure.
The second most common plasma proteins are . There are three main subgroups known as alpha, beta, and gamma globulins. The alpha and beta globulins also serve as transport “barges” as they bind ions and other molecules such as iron, lipids, and fat-soluble vitamins A, D, E, and K. Like albumin, they also contribute to blood colloid osmotic pressure. The gamma globulins are proteins involved in immunity and are better known as or . Although other plasma proteins are produced by the liver, specialized leukocytes, known as plasma cells, produce immunoglobulins. Globulins make up approximately 38 percent of the total plasma protein volume in 1.0–1.5 g/dL blood clinical levels.
The third plasma protein is . It is essential for blood clotting. Fibrinogen accounts for about 7 percent of the total plasma protein volume, in 0.2–0.45 g/dL blood clinical levels.
Plasma solutes
In addition to proteins, plasma contains a wide variety of other substances. These include various electrolytes, such as sodium, potassium, and calcium ions; dissolved gases, such as oxygen, carbon dioxide, and nitrogen; various organic nutrients, such as vitamins, lipids, glucose, and amino acids; and metabolic wastes. All of these nonprotein solutes combined contribute approximately 1 percent to the total volume of plasma.
18.2 Hemopoiesis
![]() 18.2 Learning Outcomes
18.2 Learning Outcomes
- Trace the generation of the formed elements of blood from bone marrow stem cells
- Discuss the role of hemopoietic growth factors in promoting the production of the formed elements
- Discuss bone marrow sampling and transplants
The lifespan of the most (primarily red blood cells) is relatively short. Although one category of leukocyte (s) can survive for years, most erythrocytes, leukocytes, and platelets normally live only a few hours to a few weeks. Thus, the body must form new blood cells and platelets quickly and continuously. The process by which this replacement occurs is called , or hematopoiesis (from the Greek root haima- = “blood”; -poiesis = “production”). Hemopoiesis occurs in the red marrow, a connective tissue within the spaces of spongy (cancellous) bone tissue. In children, red bone marrow is found within most bones, however, as a person ages, the red bone marrow in the appendicular skeleton is slowly replaced with yellow bone marrow (fat). In adults, the red bone marrow is largely restricted to the cranial and pelvic bones, the vertebrae, the sternum, and the proximal epiphyses of the femur and humerus. Throughout adulthood, the liver and spleen maintain their ability to produce formed elements.
All formed elements arise from stem cells of the red bone marrow. Stem cells undergo mitosis plus is (cellular division) to give rise to new daughter cells: One of these remains a stem cell and the other differentiates into one of any number of diverse cell types. Hemopoiesis starts with hemopoietic (hematopoietic) stem cells known as s. These are multipotent cells that can differentiate and become different kinds of blood cells. All of the formed elements of blood originate from this specific type of cell.
Hemopoiesis begins when the hemocytoblasts are exposed to appropriate chemical stimuli collectively called , which prompt hemocytoblasts to divide and differentiate. During this process, one daughter cell remains a , allowing hemopoiesis to continue. The other daughter cell becomes either of two types of more specialized stem cells (Figure below):
- give rise to all the other formed elements, including erythrocytes; s that produce platelets; and a myeloblast lineage that gives rise to and three forms of : , , and .
- give rise to a class of leukocytes known as , which include the various T cells, B cells, and , all of which function in immunity.

Figure 18.4: Hematopoietic System of Bone Marrow https://openstax.org/books/anatomy-and-physiology/pages/18-2-production-of-the-formed-elements#fig-ch19_02_01
| Substance | Growth Factor or Hormone | Function |
| Erythropoietin (EPO) | Hormone secreted by the kidneys | Increases the production of erythrocytes |
| Growth factor | Stimulates the development of megakaryocytes into platelets | |
| Colony-stimulating factors (CSFs) | Growth factor | Some CSFs trigger the differentiation of myeloblasts into granular leukocytes, namely, neutrophils, eosinophils, and basophils. Other CSFs induce the production of monocytes |
| Growth factor | Stimulates hemopoiesis, inflammation |
Table 18.2 Types of Hemopoietic Growth Factors.
Bone Marrow Sampling and Transplants
Sometimes, a healthcare provider will order a , a diagnostic test of a sample of red bone marrow, or a , a treatment in which a donor’s healthy bone marrow—and its stem cells—replaces the faulty bone marrow of a patient. These tests and procedures are often used to assist in the diagnosis and treatment of various severe forms of anemia, such as major and , as well as some types of cancer, specifically .
Unlike the traditional way of bone marrow examination that uses a needle and syringe to sample marrow directly from the inside of bones, the newer method of peripheral blood stem cell (PBSC) donation is a less invasive way of collecting blood-forming cells for testing. Since the same blood-forming cells that are found in bone marrow are also found in the circulating (peripheral) blood, these stem cells can be isolated in just a few hours from a sample of a patient’s blood. The isolated stem cells are then grown in culture using the appropriate hemopoietic growth factors and analyzed or sometimes frozen for later use.
For an individual requiring a transplant, a matching donor is essential to prevent the immune system from destroying the donor cells—a phenomenon known as tissue rejection. To treat patients with bone marrow transplants, it is first necessary to destroy the patient’s own diseased marrow through radiation, chemotherapy, or both. Donor bone marrow stem cells are then intravenously infused. From the bloodstream, they establish themselves in the recipient’s bone marrow.
18.3 Red Blood Cells
![]() 18.3 Learning Outcomes
18.3 Learning Outcomes
- Describe the anatomy of erythrocytes
- Discuss the various steps in the lifecycle of an erythrocyte
- Explain the composition and function of hemoglobin
- Understand the process of red blood cell formation and recycling
- Explain different causes/types of anemia
- Explain natural and exogenous ways of increasing red blood cell production, including blood doping and the possible ramifications of blood doping
Red blood cells (RBCs, also erythrocytes) are considered some of the most specialized cells in the body. Their primary function is the transportation of gasses (both oxygen and carbon dioxide) throughout the body’s blood vessels. The components of red blood cells have the unique ability to liberate (unload) oxygen off in peripheral tissues where it is needed and bind oxygen in the pulmonary capillaries. At the same time, red blood cells bind carbon dioxide in the peripheral tissues and release the CO2 in the pulmonary capillaries. Keep in mind that hydrophobic oxygen gas is not soluble in the watery matrix of plasma. If red blood cells did not exist, animal life on this planet as we know it would be vastly different. Human tissue oxygen demands are well above the amount of oxygen that could be delivered simply by blood plasma. Therefore, the ability of the red blood cells to transport both oxygen and carbon dioxide gasses is essential to most animal life, including humans.
Erythropoiesis
Red blood cell (RBC) formation and maturation begins in the red bone marrow and progresses through different stages. Recall that hemocytoblasts are hematopoietic stem cells that can differentiate into two broad types of cells; lymphocytes and all other blood cells. When the hemocytoblast differentiates into a myeloid stem cell under specific , they can further differentiate into white blood cells, megakaryocytes, or red blood cells. For example, erythropoietin (EPO) is a hormone released by the kidneys that stimulate the myeloid stem cell to differentiate into what will eventually become a mature red blood cell.
Most of the red blood cell maturation process takes place in the red bone marrow. Here the hormone erythropoietin (EPO) triggers an increased percentage of myeloid stem cells to differentiate into s. As more erythroblasts emerge, the succession of steps that convert a proerythroblast (early red blood cell) to a mature red blood cell accelerates, and more RBCs are formed.
The process of maturation of an RBC involves producing millions of hemoglobin molecules. As the cell fills with hemoglobin, they continue to shed most of their organelles, including the nucleus, to make room for the tightly packed gas-binding proteins. Once the nucleus is shed, the early RBCs, known as s, are released from the bone marrow into the circulation. Since a reticulocyte still retains some of its mitochondria, ribosomes, and endoplasmic reticulum, it can produce approximately 80 percent of the total amount of its hemoglobin molecules. Under certain histological stains, reticulocytes appear granular as they still retain some of the RNA needed to produce the remaining 20 percent of their hemoglobin. Within 48 hours of being released into the bloodstream, the reticulocyte has completed transcription and translation of the total amount of hemoglobin molecules. They shed the remaining RNA and are considered mature red blood cells. In a healthy individual, reticulocytes represent approximately 0.8 percent of circulating RBCs.
Erythrocyte Structure
Erythrocytes are biconcave discs; that is, they are plump at their periphery and very thin in the center (Figure below). Since they lack most organelles, there is more interior space for hemoglobin molecules that transport gasses. The biconcave shape also provides a greater surface area across which gas exchange can occur, relative to its volume. In narrow blood vessels called capillaries, the oxygen carried by the erythrocytes can diffuse into the plasma and then through the capillary walls to reach the cells in tissues supplied by capillaries. Whereas carbon dioxide, a waste product gas produced by cell metabolism, can diffuse into the capillaries to be picked up by the erythrocytes. Capillary beds are extremely narrow, slowing the passage of the erythrocytes and providing an extended opportunity for gas exchange to occur. However, the space within capillaries can be so minute that, despite their own small size, erythrocytes may have to fold in on themselves if they are to make their way through. Fortunately, RBC structural proteins like spectrin are flexible, allowing them to bend over themselves to a surprising degree, then spring back again when they enter a wider vessel. In wider vessels, erythrocytes may stack up much like a roll of coins, forming a , from the French word for “roll.”

Figure 18.5 Shape of Red Blood Cells https://openstax.org/books/anatomy-and-physiology/pages/18-3-erythrocytes#fig-ch19_03_02
Hemoglobin
Hemoglobin is a protein responsible for transporting oxygen and carbon dioxide. Each hemoglobin molecule has four polypeptides known as s. Two globins are called alpha chains and the other two are the beta chains. All goblins have a group, which consists of a molecular ring structure and a ferrous iron ion (Fe2+) in its center. Oxygen binds to the iron to be transported in the blood. Each molecule of hemoglobin is capable of binding four oxygen molecules. The binding of oxygen is relatively weak allowing for quick attachment of oxygen with hemoglobin when the red blood cells are passing through the pulmonary capillaries. There is also a fast detachment of oxygen when the red blood cells pass through the systemic capillaries within the tissues of the body.
Carbon dioxide binds to the globin molecule of hemoglobin. Carbon dioxide also presents a weak attachment to hemoglobin. It binds to hemoglobin as blood is moving through the systemic capillaries within the tissues of the body and is detached as blood passes through the capillaries within the lungs. In the Figure below (link to Figure Structure of hemoglobin) you will see a picture of a molecule of hemoglobin. Besides oxygen and carbon dioxide, you may be surprised to find that carbon monoxide (CO) can also bind to hemoglobin. In fact, carbon monoxide has a stronger affinity than oxygen when binding to hemoglobin. Carbon monoxide poisoning is one of the most common forms of air poisoning in many countries. Luckily, this binding of carbon monoxide is reversible if patients are moved toward a well-ventilated area with fresh air.

Figure 18.6: Structure of Hemoglobin https://openstax.org/books/anatomy-and-physiology/pages/18-3-erythrocytes#fig-ch19_03_03]
Anemia is a condition where the number of erythrocytes is lower than normal. As a result of the reduction in the number of these cells, there is a reduction in the delivery of oxygen to the cells of the body. Common symptoms of anemia include fatigue and lethargy. There are many types of anemia, so treatment of anemia depends on the causes. For some, anemia may be treated with the administration of erythropoietin, which facilitates the production of erythrocytes by the person’s own bone marrow.
Sickle cell disease is an autosomal recessive disease that leads to anemia. It happens when someone inherits two copies of the sickle cell variant (allele) of one of the genes that codes for hemoglobin. The sickle cell variant is a mutation in one of the genes that encode the polypeptides needed for hemoglobin. The mutation causes a change in the amino acid of the polypeptide chain, and patients with sickle cell disease have a faulty beta chain which affects the overall structure of the hemoglobin. Instead of a globular shape, the hemoglobin (Hb-S) has a long and slender shape. As a result of that, the erythrocytes assume a sickle or crescent shape, which prevents them from flowing through the blood vessels to the tissues of the body. In addition, these red blood cells rupture easily and they do not have the normal half-life as the regular red blood cells. This condition causes a variety of serious problems from anemia and painful joints, to delayed growth, blindness, and even cerebrovascular accidents (strokes). A picture of a sickle cell is found below.

Figure 18.7: Sickle Cells
https://openstax.org/books/anatomy-and-physiology/pages/18-3-erythrocytes#fig-ch19_03_05 ]
![]() Food and Environment: Iron Rich Food
Food and Environment: Iron Rich Food
A hemoglobin molecule has four heme groups, each with iron in its center. Iron is an essential mineral that needs to be obtained from the food that we eat. Food has two types of iron; heme and nonheme iron. Meat, fish, and poultry contain both heme and non-heme iron, while plant-based foods such as fruits, vegetables, and nuts contain nonheme iron only. Heme irons are more readily absorbed (up to 30 percent of what you consume) than the non-heme irons which are absorbed between two to ten percent of what you consume. That does not mean that we should be choosing to only eat meat as a source of iron. Heme iron is absorbed better when foods containing heme iron are consumed with foods higher in nonheme iron. That means to get the maximum iron absorption, you should eat your vegetables with your steak! Some of our favorite foods that are iron-rich include: lean meat, eggs, liver and liverwurst, shrimp, tuna, sardines, tofu, beans, dark-colored vegetables such as spinach, kale, beet greens, broccoli, string beans, sweet potatoes, strawberries, and watermelon, raisins, prunes. Even more, vitamin C increases the absorption of iron. Infants, young children, teenage girls, pregnant women, and premenopausal women are at risk of obtaining insufficient amounts of iron from their diets. So, next time you host a birthday party or a baby shower, consider serving a sweet potato salad or a big platter of strawberries, watermelon, and oranges, along with grilled garlic shrimp or short ribs to make it an iron-rich feast!
Food rich in Heme Iron and Nonheme Iron. Meat, fish, and poultry contain both heme and nonheme irons. Plant-based foods are rich in nonheme iron.
Erythropoiesis
Erythropoiesis is the process of the formation of new red blood cells. Keep in mind that to stay healthy a person’s production of RBCs needs to keep pace with the number of RBCs destroyed and recycled. Erythropoiesis is stimulated by the peptide hormone erythropoietin (EPO). EPO has a direct effect on the stimulation and production of new RBCs. Other hormones such as androgens, thyroid hormone, and growth hormone have indirect effects in stimulating the proliferation of RBCs. Erythropoietin is produced by the kidneys and to a smaller extent, the liver. EPO is produced and released by the kidneys in conditions. If the body tissues are starved of oxygen, the body’s reaction is to increase the ability of blood to transport more oxygen by producing more oxygen-carrying entities – the red blood cells. Therefore, when tissue is starved of oxygen, EPO is released into the bloodstream. Here it travels to the red bone marrow and stimulates the myeloid stem cells to further differentiate into proerythroblasts. This increase in the rate and speed at which these cells are made can drastically increase the rate at which immature RBCs (reticulocytes) are released into the circulation. The Figure below shows the erythropoietin regulation of the production of erythrocytes (see Figure 18.8 Erythropoietin).
Hypoxic conditions can result from various forms of anemia, blood loss, and changes in altitude. You have probably heard of vitamin B12, which is a critical vitamin for nucleotide synthesis for the body, and especially for red blood cell formation. For example, if a person has inadequate dietary absorption of vitamin B12, normal stem cell divisions decline and the number of red blood cells in circulation suffers. A decline in the number of RBCs leads to a decline in the oxygen-carrying capacity of the blood. This type of anemia previously described is known as pernicious anemia. Pernicious anemia is normally due to a lack of intrinsic factors made by parietal cells in the stomach. Intrinsic factor is critical for the absorption of vitamin B12 in the GI tract. If there are inadequate amounts of intrinsic factors produced, it will lead to inadequate amounts of vitamin B12 being absorbed, decreased RBC production, and thus, a decreased ability for the blood to carry adequate amounts of oxygen.
![]() Cultural Connection
Cultural Connection
Many local endurance athletes take advantage of the high altitudes offered in Hawai’i to naturally increase their EPO levels. The tops of large mountains like Haleakalā, Mauna Kea, or Mauna Loa have a thinner atmosphere and less partial pressure of oxygen than at sea level. This low-oxygen environment provides an optimal environment for inducing the natural release of EPO (see the callout box).
Under normal conditions, a healthy individual is releasing approximately three million new red blood cells into circulation each second. Under maximal EPO stimulation (i.e., taking exogenous EPO) that number can increase tenfold to 30 million each second! However, in the case of the number of red blood cells, more is not always better. In fact, too much can be harmful to your health. Supplementing with exogenous EPO will lead to a dramatic increase in the hematocrit level. As the hematocrit level increases, the blood becomes thicker and more viscous, which slows down blood flow (a phenomenon called resistance). The increase in blood viscosity, in turn, creates more resistance in the vessel. This causes a dramatic increase in stress on the heart, which has to work and pump harder to overcome the increased resistance. This burden on the heart due to too much EPO and RBCs can lead to increased risks of heart disease, stroke, and pulmonary embolism.
![]() Clinical Application
Clinical Application
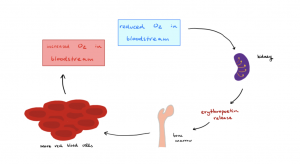
Figure 18.8 Erythropoietin https://commons.wikimedia.org/wiki/File:Erythropoietin_control_of_erythrocyte_number.png]
Blood Doping
Another dangerous activity that some endurance athletes engage in is the process of blood doping. Blood doping is the process of extracting blood at an earlier date, separating the formed elements from the plasma, and then reinfusing the formed elements to boost hematocrit levels. Again, this is a dangerous activity as it also increases the viscosity of the blood and thus, the workload of the heart.
RBC Recycling
Out of the estimated 30-40 trillion cells that make up the human body, over half are ! As stated earlier, red blood cells cannot repair themselves when damaged. As RBCs mature, they shed their organelles (including their nucleus!) to make room for hemoglobin molecules and therefore do not have the internal machinery involved to render repairs. During the lifetime of a typical red blood cell (120 days), the cell is exposed to various stresses as it makes its way through the circulatory system. RBCs are damaged as they rebound off vessel walls, other cells, and heart valves. RBCs also encounter mechanical stresses as they are squeezed through narrow capillaries. As RBCs circulate throughout the cardiovascular system, the cell and its membrane become damaged and eventually undergo (rupture). s in various tissues recognize damaged RBCs and typically engulf them before they hemolyze. A small percentage of RBCs meet their demise in the general circulation and not at the mouths of macrophages. Once ruptured, specific cell components are recycled as they are vital and not readily replaced. Appreciate that roughly three million new red blood cells are recycled each second. In a healthy individual, the number of new red blood cells entering the circulation must match the number of red blood cells that rupture.
Recycling and eliminating different components of the vast number of ruptured red blood cells is essential to maintaining homeostasis. It should be noted that there are organs involved with the circulatory system that specialize in the recycling of red blood cells. They are the liver, red bone marrow, and most importantly, the spleen. The spleen is an organ that is considered part of the lymphatic system, but it also functions as the graveyard for damaged red blood cells. Macrophages engulf most damaged red blood cells in the spleen as they squeeze through the narrow, convoluted splenic blood vessels.
Recall that each RBC contains roughly 280 million hemoglobin molecules. Each heme molecule is composed of four separate chains of polypeptides that form a protein subunit containing a single molecule of heme bound to an iron ion in its ferrous form (Fe2+). The iron portion of the heme is essential, and the iron must be recycled and the heme portion (a waste product) be eliminated. The body has devised a clever way to eliminate the heme portion of hemoglobin while at the same time retaining and reusing the iron portion. Let us first trace the journey of recycled iron. Once a macrophage digests damaged red blood cells, they release the iron back into the bloodstream. Here, the iron is picked up by a plasma protein called , which transports and circulates back to the bone marrow, where it delivers iron, which is used to produce more hemoglobin molecules.
Heme molecules encounter a more interesting fate. They are considered a waste product and need to be eliminated from the body. For this process to occur, the macrophages that phagocytosed and digested the blood cells first convert the heme molecules to a greenish compound known as , which is then converted to (a yellowish pigment) and released into the bloodstream. This conversion can be seen when a changes colors from a dark purple-red color and changes to greenish yellow as the macrophages clear and convert red heme into biliverdin and bilirubin. Cells of the liver pick up and collect circulating bilirubin molecules and secrete this waste product into the small intestine as a component of bile. The bilirubin makes its way into the large intestine, where intestinal bacteria convert it to components known as and . Urobilins and stercobilins contribute to urine and fecal matter’s typical brown color and contribute partially to its odor, respectively. Furthermore, a small number of urobilins are absorbed by the large intestine into the bloodstream and eventually eliminated in the urine by the kidneys. This contributes to urine’s yellowish color.

Figure 18.9: Erythrocyte Lifecycle https://openstax.org/books/anatomy-and-physiology/pages/18-3-erythrocytes#fig-ch19_03_04
In certain conditions, bilirubin can build up in the circulatory system, giving the person a yellowish hue, which is a condition called . For example, this may happen when there is an impaired liver function, such as or a blockage of the common bile duct by a gallstone. In this instance, the liver is either not eliminating adequate amounts of bilirubin in the bile, or the bile duct is blocked. As a result, bilirubin leaks into the general circulation. Other conditions or diseases where the person experiences a vast amount of red blood cell destruction in a short amount of time can also lead to jaundice.
![]() Retrieval Practice: Erythrocyte Life Cycle
Retrieval Practice: Erythrocyte Life Cycle
18.4 White Blood Cells
![]() 18.4 Learning Outcomes
18.4 Learning Outcomes
- Describe the general characteristics of leukocytes
- Classify leukocytes according to their lineage, their main structural features, and their primary functions
- Discuss the most common disorders involving leukocytes
have a hugely different function compared to red blood cells. White blood cells do not have hemoglobin, therefore, they are not involved with the transportation of gases. They exist to help fight infection. When white blood cells were first discovered, they were named based on their appearance. A stain was used to better observe these cells and the cells that appeared granular were appropriately named granulocytes while the population of white blood cells that did not appear grainy were called agranulocytes. There are three types of granulocytes: neutrophils, eosinophils, and basophils. The two types of agranulocytes are monocytes and lymphocytes. Although white blood cells are categorized by the presence or absence of a granular appearance, the categorization describes little about each function. The individual functions will be discussed quickly in this chapter, with more detail in the immunity chapter.

Figure 18.10 Granular Leukocytes https://openstax.org/books/anatomy-and-physiology/pages/18-4-leukocytes-and-platelets#fig-ch19_04_02

Figure 18.11: Agranulocytes (lymphocytes and monocytes)

 |
 |
 |
 |
 |
| Basophil | Eosinophil | Neutrophil | Monocyte | Lymphocyte |
Figure 18.12 Histology images (microscopic images) of all white blood cells.
Red blood cells vastly outnumber the white blood cells in the blood. In a healthy individual, there is about only one white blood cell for every 1000 red blood cells. However, most white blood cells are not circulating in the blood. Instead, they are found roaming through connective tissue or circulating through the lymphatic system.
Leukocyte Characteristics
One can think of the bloodstream as a delivery system for white blood cells. Unlike red blood cells, white blood cells do not spend their entire life circulating throughout the bloodstream. Instead, WBCs can leave the bloodstream when stimulated by certain chemicals released from injured tissue. Some WBCs live only a few days, but in instances where they are actively fighting infection, their lifespans are longer. In addition, we will learn about memory cells which have long lifespans that last for decades.
All white blood cells display certain characteristics. As stated earlier, WBCs can leave the bloodstream when stimulated. Chemicals released into the bloodstream by damaged tissue and other white blood cells attract the circulating white blood cells in the area and trigger them to become sticky like velcro and attach to the inside of the endothelial lining of capillaries. This chemical attraction is called . Like chum placed in the ocean to attract sharks, these chemicals released into the blood from this tissue damage causes the white blood cells to become attracted to the area. When in the area, WBCs undergo a process called , where they grasp the inside endothelial layer of the vessel wall and squeeze themselves through the narrow cracks in the capillary endothelium. Once out of the bloodstream, WBCs can move through tissue. The act of moving is an active process that requires ATP and calcium ions allowing actin filaments to contract along the cytoskeleton of the cell. The constant rearrangement and contraction of actin filaments allow the cell to propel itself through tissue. This is called amoeboid movement (they move similar to how an amoeba moves). Finally, certain WBCs have the ability to phagocytose substances they deem as foreign to the body.

Figure 18.13: Emigration [https://openstax.org/books/anatomy-and-physiology/pages/18-4-leukocytes-and-platelets#fig-ch19_04_01 ]
Leukopoiesis
The production of all blood cells (including RBCs) takes place in the bone marrow. The hematopoietic stem cell that gives rise to all blood cells is the hemocytoblast. In the bone marrow, hemocytoblasts (under the influence of various colony-stimulating factors) will give rise to two distinct subclasses of cells, the myeloid stem cell and the lymphoid stem cell. The myeloid stem cell, under the influence of hormones or colony-stimulating factors, will further differentiate into cells that mature into RBCs, megakaryocytes, basophils, eosinophils, neutrophils, or monocytes. If the hemocytoblast differentiates into lymphoid stem cells the outcome will be various types of lymphocytes.
The five types of leukocytes, or white blood cells (WBCs), are neutrophils, eosinophils, basophils, monocytes, and lymphocytes. Leukopoiesis is the term used to generally describe the formation of all of these types of leukocytes.
Neutrophils
We will start our discussion with the most abundant white blood cell found in the circulation. Neutrophils are considered granulocytes. In a healthy individual, neutrophils make up approximately 50-70 percent of circulating white blood cells. Neutrophils are significantly larger than a RBC (10-12 μm in diameter). As the neutrophil ages, its nucleus develops multiple lobes. Young neutrophils start with a two-lobed nucleus, but as they mature can develop upwards of 5 lobes. Because they are multi-lobed in appearance they are known as . As the first responders in a traffic accident, neutrophils are very mobile and are typically the first white blood cells at the scene of damaged tissue. Here they become extremely aggressive at phagocytosing bacteria and damaged tissue. Their granules contain an enzyme able to lyse (break apart) cell membranes known as . In addition, they contain that are also able to damage the cell membranes of bacteria and fungus. When stimulated, neutrophils aggressively phagocytose bacteria and damaged tissue. While doing so they release chemicals that affect local inflammation and serve as chemotaxis attracting other leukocytes to the area. They eventually “eat” themselves to death. A collection of dead white blood cells and damaged tissue is referred to as pus.
Neutrophils are specialists at phagocytosing bacteria and tend to increase dramatically in number when a person is actively fighting off a bacterial infection. They also increase dramatically in individuals with severe burns. Burns on the skin decrease the skin’s effectiveness as a barrier to invading bacteria, triggering the increase in the bacteria-fighting specialists, the neutrophils.
Eosinophils
Eosinophils are much less numerous in the bloodstream of a healthy individual. They represent approximately 2-4 percent of circulating WBCs. They received their name as their granules take up the stain eosin which has a rust orange color. Eosinophils also have a multilobed nucleus with typically 2-3 lobes. They are roughly the same size as the aforementioned neutrophil. Although eosinophils have the ability to phagocytose targets that are coated with antibodies, they have another interesting mechanism for killing pathogens. Eosinophil granules contain chemicals that are toxic to large multicellular organisms that are far too large to engulf. For example, it would be impossible for a single cell to engulf a large parasite such as a roundworm. In this case, the eosinophil releases nitric oxide and cytotoxic enzymes that destroy the integrity of the body wall of such parasites, killing them in the process. Eosinophils will increase in number during allergic reactions, parasitic worm infections, and certain autoimmune diseases.
Basophils
Basophils are granular leukocytes filled with vesicles containing histamine and . They are slightly smaller than the neutrophil or eosinophils with a diameter of 8-10 μm. In a healthy individual, these cells represent only approximately one percent of circulating WBCs. When stimulated, these cells release their vesicles of histamine and heparin. Histamine acts to stimulate the inflammatory process, and heparin prevents blood clotting, allowing more blood flow into the injured area. It should be noted that it was once believed that mast cells of the connective tissue were basophils that had migrated to that area. It is now known that these two cells, although similar in function, have different origins.
Monocytes
Monocytes are considered and represent approximately two to eight percent of circulating leukocytes. Monocytes are by far the largest of the leukocytes. They are easily distinguishable by their size at 12-20 μm. When viewed under a microscope, monocytes appear very large and typically display a kidney-bean shaped nucleus. Monocytes end up leaving the bloodstream after circulating for a short period (within 24-48 hrs). Once in peripheral tissues, they grow even larger and differentiate into large phagocytic cells known as macrophages. Upon stimulation, macrophages, as their name implies, can engulf large amounts of foreign debris. While doing so they release chemotactic chemicals in the area that attracts other leukocytes. Macrophages are considered fixed or free based on their mobility throughout tissues. Fixed macrophages remain in the local area, whereas free macrophages meander throughout various tissues of the body engulfing substances they deem as foreign. Abnormally high counts of monocytes are associated with viral or fungal infections, tuberculosis, and some forms of leukemia and other chronic diseases. Abnormally low counts are typically caused by suppression of the bone marrow.
Lymphocytes
The final type of agranulocyte is the lymphocyte. When observed under a slide, lymphocytes appear to be just slightly larger than a RBC. The nucleus occupies most of the space within the cell with typically just a slight sliver of cytoplasm observed between the nucleus and cell membrane. Lymphocytes are unique in the fact that they are the only blood cells that arise from lymphoid stem cells. All other formed elements arise from a stem cell known as a myeloid stem cell. Although the circulating percentage of lymphocytes is around 20-40 percent of the total circulating leukocytes, it should be noted that there are far more lymphocytes found migrating throughout lymphatic and peripheral tissues. There are three main groups of lymphocytes: the NK (natural killer) cells, , and .
Natural killer cells (also, NK cells) are usually larger than T or B lymphocytes and can provide an important innate immune function known as immune surveillance. Immune surveillance is a process of recognizing the surface markers on various cells and detecting abnormal or infected cells. Cells have glycoprotein combinations protruding from their cell membranes acting like a flag that designates the cell as “self”. However, a cancerous, damaged, or virus-infected cell will not display these healthy “self” surface markers. The NK will detect these damaged cells and conduct steps to destroy these cells.
T lymphocytes (T cells) are essential to carry out a process known as cell-mediated immunity. This is considered part of an individual’s adaptive immunity (also, specific immunity) that recognizes foreign molecules. There are also many subcategories of T cells. B lymphocytes (B cells) are responsible for a process known as humoral immunity, a process by which they are stimulated to proliferate and produce antibodies that can bind and attack foreign pathogens throughout the body. There are also subcategories of B cells. The types and functions of these cells will be discussed further in the immunity chapter.

Figure 18.14 Summary of Formed Elements in Blood [https://openstax.org/books/anatomy-and-physiology/pages/18-3-erythrocytes#fig-ch19_03_01 ]
Disorders of Leukocytes
Because of the important immune functions provided by leukocytes, disorders of these cells can have devastating consequences. Maintaining healthy ratios of these cells is important in providing adequate immune responses. If there is a condition where too few white blood cells are produced it is known as . If leukopenia is severe enough, it may leave the person immunocompromised, leaving them susceptible to certain diseases. If there is a proliferation of the WBC count it is known as . In many instances, leukocytosis can also leave the individual immunocompromised. Even though there is an overall increase in the number of WBCs in leukocytosis, the cells may not be functional and leave the person unable to fight off disease.
Extreme leukocytosis typically indicates a condition known as leukemia. This is cancer involving the myeloid line or lymphoid line of cells where abnormal leukocytes proliferate and divide at abnormally high rates. Leukemia can be divided into two general categories. Acute leukemia and chronic leukemia. In acute leukemia, there is a rapid proliferation of immature blood cells. This type of leukemia is common amongst younger individuals and leads to overcrowding of non-functional blood cells, reducing the oxygen-carrying capacity of blood and its ability to clot. An additional danger of this type of leukemia is the fact that these malignant cancer cells may spread to other tissues and organs of the body. Chronic leukemia leads to the excessive buildup of non-functional WBCs and is a much slower progressing disorder that is more common in older individuals.
18.5 Platelets
![]() 18.5 Learning Outcomes
18.5 Learning Outcomes
- Identify and describe the lineage, basic structure, and function of platelets
Platelets are also sometimes called . However, “thrombocyte” (with cyte in the suffix) can be slightly misleading as it infers that platelets are cells. However, some medical terms like and describe abnormal amounts of platelets, so it is still useful to know the terms.
Platelets are not entire cells, but instead are fragments produced from the shedding of very large cells known as megakaryocytes. Each megakaryocyte throughout its lifespan will shed 2000-3000 of these fragments, which are all enclosed by part of the original megakaryocyte’s cell membrane. Once shed, these fragments are considered individual platelets. Platelets are the smallest of the formed elements. Platelets are smaller than RBCs and have a width of about 2-4 μm. Once released into circulation, they only circulate for roughly 10 days before they are phagocytosed by macrophages. Platelets are involved with blood clotting, and therefore critical to , the ability for a ruptured blood vessel to form a clot.

Figure 18.15 Platelets [https://openstax.org/books/anatomy-and-physiology/pages/18-4-leukocytes-and-platelets#fig-ch19_04_03 ]
18.6 Hemostasis
![]() 18.6 Learning Outcomes
18.6 Learning Outcomes
- Describe the steps and mechanisms involved in hemostasis
- Explain how the extrinsic and intrinsic coagulation pathways lead to the common pathway, and the coagulation factors involved in each
When injury to a blood vessel occurs, the process of hemostasis is triggered. This process is initiated to stop the bleeding. This process consists of three overlapping phases: , formation of the , and (blood clotting).
Vascular Spasm
In this phase, the smooth muscle within the wall of the blood vessel contracts, which results in vasoconstriction and a reduction in the amount of blood that is leaking from the vessel. This constriction is called a vascular spasm and only lasts for a few minutes.
Formation of the Platelet Plug
When a blood vessel is damaged, there is a formation of a platelet plug. This occurs due to the damage of the vessels and results in exposure of underlying collagen fibers within the connective tissue in the endothelial wall. As a result, platelets start to stick to the collagen fibers with the help of von Willebrand factor, a plasma protein. As more and more platelets adhere to the wall of the vessel, they form long processes that stick to the vessel wall. Consequently, there is a formation of the platelet plug that closes off the damaged site. In this process, the cytosol of the platelets degranulates and releases chemicals. In response to these chemicals, several processes will happen: 1) the vascular spasms and smooth muscle contraction will continue due to the release of serotonin and thromboxane A2; 2) other platelets will be attracted to the damaged site due to the release of adenosine diphosphate (ADP); 3) Coagulation will be triggered with the release of procoagulants; and 4) some of the substances released by the platelets stimulate the tissue and fibroblasts to replicate, which stimulates the repair of the blood vessel.
The process of formation of the platelet plug is an example of positive feedback. In order to prevent the unnecessary increase of the platelet plug beyond the injury site, the endothelial cells localized in the inner lining of the blood vessel, produce prostacyclin that ultimately inhibits the activation of platelets and the plug does not grow beyond the area of the injury.
Coagulation
Coagulation is the process of clotting of the blood. The blood clot contains , which is an insoluble protein derived from fibrinogen. Fibrin forms a meshwork that traps the formed elements of the blood in the process of clot formation. Examples of the many substances that are part of this process are found in Table 18.3 below. Most are inactive enzymes produced by the liver.
| Factor number | Name | Type of molecule | Source | Pathway(s) |
| I | Fibrinogen | Plasma protein | Liver | Common; converted into fibrin |
| II | Prothrombin | Plasma protein | Liver* | Common; converted into thrombin |
| III | Tissue thromboplastin or tissue factor | Lipoprotein mixture | Damaged cells and platelets | Extrinsic |
| IV | Calcium ions | Inorganic ions in plasma | Diet, platelets, bone matrix | Entire process |
| V | Proaccelerin | Plasma protein | Liver, platelets | Extrinsic and intrinsic |
| VI | Not used | Not used | Not used | Not used |
| VII | Proconvertin | Plasma protein | Liver * | Extrinsic |
| VIII | Antihemolytic factor A | Plasma protein factor | Platelets and endothelial cells | Intrinsic; deficiency results in A |
| IX | Antihemolytic factor B (plasma thromboplastin component) | Plasma protein | Liver* | Intrinsic; deficiency results in hemophilia B |
| X | Stuart–Prower factor (thrombokinase) | Protein | Liver* | Extrinsic and intrinsic |
| XI | Antihemolytic factor C (plasma thromboplastin antecedent) | Plasma protein | Liver | Intrinsic; deficiency results in hemophilia C |
| XII | Hageman factor | Plasma protein | Liver | Intrinsic; initiates clotting in vitro also activates plasmin |
| XIII | Fibrin-stabilizing factor | Plasma protein | Liver, platelets | Stabilizes fibrin; slows fibrinolysis |
Table 18.3 Clotting Factors [https://openstax.org/books/anatomy-and-physiology/pages/18-5-hemostasis#tbl-ch19_01]
There are two mechanisms involved in the initiation of blood clotting: the and the . The intrinsic pathway is more complex and it takes longer than the extrinsic pathway. However, both of these pathways converge on a .
The injury to the inside of the wall of the blood vessel triggers the intrinsic pathway. The following events happen:
- Factor XII comes into contact with foreign materials,
- Factor XII sets off a series of reactions that in turn activates factor XI then factor IX,
- Chemicals released by the platelets increase the rate of these activation reactions,
- Factor VIII from the platelets and endothelial cells combines with factor IX,
- The formation of an enzyme complex that activates factor X, leads to the common pathway.
- The events in the intrinsic pathway are completed in a few minutes.
When the damage happens to the outside of the blood vessel, the extrinsic pathway is triggered. In this shorter pathway, 1) combines with factor VII and calcium forming a complex; 2) Inactive factor X is converted into the active factor X. Factor X is the first step in the common pathway and could be initiated by either the intrinsic pathway or extrinsic pathway:
- The enzyme prothrombinase converts factor II, the inactive enzyme prothrombin, into the active enzyme ,
- Thrombin converts factor I, the soluble fibrinogen, into the insoluble fibrin protein strands,
- Factor XIII stabilizes the fibrin clot.
Fibrin is then formed and the formed elements become trapped within the meshwork. Blood clotting is also an example of positive feedback. Once the clot, also known as a , is formed, the events will stop.
See the Figure below that illustrates the three events of hemostasis.

Figure 18.16 Hemostasis [https://openstax.org/books/anatomy-and-physiology/pages/18-5-hemostasis#fig-ch19_05_01]
Clot Removal
Eventually, the blood clot needs to be eliminated allowing for repair of the blood vessel wall to occur. The first event that happens is the reduction of the size of the clot. Contractile proteins within the platelets, known as actin and myosin, contract and pull the sides of the vessel walls close together. The process to remove the meshwork of fibrin is called and it is accomplished by that degrades the strands of fibrin.
Disorders, such as atherosclerosis, can trigger unnecessary and potentially serious blood clotting. Atherosclerosis is the buildup of fats and cholesterol in the artery walls. This buildup is called plaque. The plaque can cause the arteries to narrow, increasing the resistance to blood flow. The plaque can also burst, leading to a blood clot.
Problems can also occur if clots detach and travel throughout blood vessels, where they can end up blocking blood vessels and blood flow. When a blockage in a blood vessel occurs, it is called an , which starves cells and tissues behind the blockage of the blood supply. An embolus can be extremely harmful and potentially deadly if it blocks blood vessels in vital organs such as the brain, heart, and lungs. Therefore, the body must have the ability to remove clots when they are no longer needed, or if a clot accidentally forms in a place it should not be.
18.7 Blood Types
![]() 18.7 Learning Outcomes
18.7 Learning Outcomes
- Explain how ABO and Rh blood type is determined, and compare and contrast ABO and Rh blood groups
- Predict which blood groups may be safely transfused into patients with different ABO types
- Describe the physiological consequences of transfusion of incompatible blood
- Discuss the pathophysiology of hemolytic disease of the newborn
The plasma membrane of erythrocytes has glycoproteins and glycolipid molecules, known as surface antigens, also called agglutinogens. Based on the presence or absence of these surface antigens, blood is classified into different blood groups. There are at least 24 blood groups and we will take a closer look at two of them: the and the Rh protein.
ABO Blood Group
The ABO group has two surface antigens, which are glycoproteins. They are called the A antigen and B antigen. A person’s ABO blood type is determined by the presence or absence of the A antigen, the B antigen or both.
- Type A blood has erythrocytes with antigen A only
- Type B blood has erythrocytes with antigen B only
- Type AB blood has erythrocytes with both antigens A and B
- Type O blood has erythrocytes with neither antigen A and B
In addition to the ABO surface antigens found on erythrocytes, the blood plasma has antibodies. An antibody is a Y-shaped protein that recognizes a specific antigen. Anti-A and anti-B antibodies recognize the surface antigen A and the surface antigen B, respectively. We do not have antibodies in our own blood plasma that bind to the surface antigens of our own erythrocytes. But we do have the antibodies that recognize those that we lack. These are the blood types and corresponding antibodies within the plasma:
- Type A blood has antigen A on the RBC and anti-B antibodies in the plasma
- Type B blood has antigen B on the RBC and anti-A antibodies in the plasma
- Type AB blood has both antigens (A and B) on the RBC and has neither anti-A nor anti-B antibodies
- Type O blood has no antigen A or B on the RBC and has both anti-A nor anti-B antibodies
Rh Factor
The Rh blood type is classified according to the presence or absence of the Rh surface antigen on the plasma membrane of erythrocytes. The Rh surface antigen is also known as Rh-factor or surface antigen D. A person is Rh-positive when the Rh factor is present. When the Rh factor is absent, a person is considered Rh-negative. The so-called anti-D antibodies or antibodies to the Rh factor occur in the blood only when a Rh-negative person is exposed to Rh-positive blood. One way that this can happen is through an improper blood transfusion. People who are Rh-positive never show anti-D antibodies due to the fact that they have the Rh antigen on their erythrocytes.
When identifying a patient’s blood type, the Rh group is designated by adding the word positive or negative to the ABO type. For example, A positive (A+) means ABO group A blood with the Rh antigen present, and AB negative (AB−) means ABO group AB blood without the Rh antigen. Although the ABO group and the Rh factor are reported together, none of them influence the presence or absence of each other. Below is a chart that summarizes the characteristics of the blood types in the ABO blood group.

Figure 18.17: ABO Blood Group https://openstax.org/books/anatomy-and-physiology/pages/18-6-blood-typing#fig-ch19_06_03
In blood transfusion, compatibility between donor and recipient has to be determined before the transfusion to avoid transfusion reactions. If the blood of an incompatible type is transfused to a person, antibodies in the plasma can recognize surface antigens of the transfused erythrocytes. As a result, clumping of the erythrocytes can occur, a process clinically known as . Several serious problems can result from agglutination, such as the clumped erythrocytes can block blood vessels and prevent blood from being delivered to the heart and brain. Destruction of erythrocytes, a process called hemolysis, can also happen and can cause damage to several organs.
It is best to transfuse only matching blood types; that is, a type A+ recipient should ideally receive blood only from a type A+ donor and so on. That said, in emergencies, when acute threatens the patient’s life, there may not be time for cross-matching to identify blood type. In these cases, blood from a —an individual with type O− blood—may be transfused. Recall that type O erythrocytes do not display A or B antigens. Thus, anti-A or anti-B antibodies that might be circulating in the patient’s blood plasma will not recognize any erythrocyte surface antigens on the donated blood and therefore will not be triggered into a response.
A patient with blood type AB+ is known as the . This patient can theoretically receive any type of blood because the patient’s own blood—having both A and B antigens on the erythrocyte surface—does not produce anti-A or anti-B antibodies. In addition, an Rh+ patient can receive both Rh+ and Rh− blood. However, keep in mind that the donor’s blood will contain circulating antibodies, again with possible negative implications. Since there are other blood antigens and antibodies that are not covered in this section, it is important to exercise caution when using the words “universal donor or recipient”. Ultimately, blood should be carefully screened or cross-matched before transfusion to avoid problems.
Blood Typing
Commercially prepared antibodies are available to determine the blood type of a person, a process called blood typing. In ABO blood typing, a blood sample from the same person is allocated into separate wells. A small amount of anti-A antibody is added to one well, and a small amount of anti-B antibody is added to another well. If the antigen is present, the antibodies will cause visible agglutination of the cells. The Figure (Figure Blood Types) below shows an example of how to determine a blood type. This sample of a commercially-produced “bedside” card enables quick typing of both a recipient’s and donor’s blood before transfusion. The card contains three reaction sites or wells. One is coated with an anti-A antibody, one with an anti-B antibody, and one with an anti-D antibody (tests for the presence of Rh factor D). Mixing a drop of blood and saline into each well enables the blood to interact with a preparation of type-specific antibodies, also called anti-. Agglutination of RBCs in a given site indicates a positive identification of the blood antigens, in this case, A and Rh antigens for blood type A+. For transfusion, the donor’s and recipient’s blood types must match.

Figure 18.18: Cross Matching Blood Types [https://openstax.org/books/anatomy-and-physiology/pages/18-6-blood-typing#fig-ch19_06_02]

Figure 18.19: Blood Typing
![]() Clinical Application
Clinical Application

Figure 18.20 Hemolytic Disease of the Newborn. (a) When an Rh− mother has an Rh+ fetus, fetal erythrocytes are introduced into the mother ’s circulatory system before or during birth, leading to production of anti-Rh IgG antibodies. These antibodies remain in the mother and, if she becomes pregnant with a second Rh+ baby, they can cross the placenta and attach to fetal Rh+ erythrocytes. Complement-mediated hemolysis of fetal erythrocytes results in a lack of sufficient cells for proper oxygenation of the fetus. (b) HDN can be prevented by administering Rho(D) immune globulin during and after each pregnancy with an Rh+ fetus. The immune globulin binds fetal Rh+ RBCs that gain access to the mother ’s bloodstream, preventing activation of her primary immune response.

Chapter Summary
Quiz
References & Attribution
- OpenStax https://openstax.org/books/anatomy-and-physiology/pages/18-2-production-of-the-formed-elements
- https://openstax.org/books/anatomy-and-physiology/pages/18-3-erythrocytes
- Bone marrow examination through Peripheral Blood Stem Cell (PBSC)
- Carbon monoxide poisoning
- Monocytes section

Key Terms
ABO blood group
blood-type classification based on the presence or absence of A and B glycoproteins on the erythrocyte membrane surface
agglutination
clustering of cells into masses linked by antibodies
agranular leukocytes
leukocytes with few granules in their cytoplasm; specifically, monocytes, lymphocytes, and NK cells
albumin
most abundant plasma protein, accounting for most of the osmotic pressure of plasma
anemia
deficiency of red blood cells or hemoglobin
antibodies
(also, immunoglobulins or gamma globulins) antigen-specific proteins produced by specialized B lymphocytes that protect the body by binding to foreign objects such as bacteria and viruses
B lymphocytes
(also, B cells) lymphocytes that defend the body against specific pathogens and thereby provide specific immunity
basophils
granulocytes that stain with a basic (alkaline) stain and store histamine and heparin
bilirubin
yellowish bile pigment produced when iron is removed from heme and is further broken down into waste products
biliverdin
green bile pigment produced when the non-iron portion of heme is degraded into a waste product; converted to bilirubin in the liver
blood
liquid connective tissue composed of formed elements—erythrocytes, leukocytes, and platelets—and a fluid extracellular matrix called plasma; component of the cardiovascular system
blood colloid osmotic pressure
created by plasma proteins, namely albumin, that do not diffuse readily across the capillary membrane and serves to hold water within the vascular space.
bone marrow biopsy
diagnostic test of a sample of red bone marrow
bone marrow transplant
treatment in which a donor’s healthy bone marrow with its stem cells replaces diseased or damaged bone marrow of a patient
bruise
localized bleeding under the skin due to damaged blood vessels
buffy coat
thin, pale layer of leukocytes and platelets that separates the erythrocytes from the plasma in a sample of centrifuged blood
Cirrhosis
a chronic disease of the liver marked by degeneration of cells, inflammation, and fibrous thickening of tissue. It is typically a result of alcoholism or hepatitis.
clotting factors
group of 12 identified substances active in coagulation
coagulation
formation of a blood clot; part of the process of hemostasis
colony-stimulating factors (CSFs)
glycoproteins that trigger the proliferation and differentiation of myeloblasts into granular leukocytes (basophils, neutrophils, and eosinophils)
common pathway
final coagulation pathway activated either by the intrinsic or the extrinsic pathway, and ending in the formation of a blood clot
cross matching
blood test for identification of blood type using antibodies and small samples of blood
cytokines
class of proteins that act as autocrine or paracrine signaling molecules; in the cardiovascular system, they stimulate the proliferation of progenitor cells and help to stimulate both nonspecific and specific resistance to disease
defensins
antimicrobial proteins released from neutrophils and macrophages that create openings in the plasma membranes to kill cells
diapedesis
(also, emigration) process by which leukocytes squeeze through adjacent cells in a blood vessel wall to enter tissues
embolus
thrombus that has broken free from the blood vessel wall and entered the circulation
Eosinophils
granulocytes that stain with eosin; they release antihistamines and are especially active against parasitic worms
erythroblast
nucleated cell occurring in red marrow as a stage or stages in the development of the red blood cell, or erythrocyte
Erythrocyte
(also, red blood cell) mature myeloid blood cell that is composed mostly of hemoglobin and functions primarily in the transportation of oxygen and carbon dioxide
erythropoietin (EPO)
glycoprotein that triggers the bone marrow to produce RBCs; secreted by the kidney in response to low oxygen levels
extrinsic pathway
initial coagulation pathway that begins with tissue damage and results in the activation of the common pathway
fibrin
insoluble, filamentous protein that forms the structure of a blood clot
fibrinogen
plasma protein produced in the liver and involved in blood clotting
fibrinolysis
gradual degradation of a blood clot
formed elements
cellular components of blood; that is, erythrocytes, leukocytes, and platelets
globin
heme-containing globular protein that is a constituent of hemoglobin
globulins
heterogeneous group of plasma proteins that includes transport proteins, clotting factors, immune proteins, and others
granular leukocytes
leukocytes with abundant granules in their cytoplasm; specifically, neutrophils, eosinophils, and basophils
hematocrit
(also, packed cell volume) volume percentage of erythrocytes in a sample of centrifuged blood
hematopoietic stem cell
type of pluripotent stem cell that gives rise to the formed elements of blood (hemocytoblast)
heme
red, iron-containing pigment to which oxygen binds in hemoglobin
hemocytoblast
hematopoietic stem cell that gives rise to the formed elements of blood
hemoglobin
oxygen-carrying compound in erythrocytes
hemolysis
destruction (lysis) of erythrocytes and the release of their hemoglobin into circulation
hemolytic disease of the newborn (HDN)
(also, erythroblastosis fetalis) disorder causing agglutination and hemolysis in an Rh+ fetus or newborn of an Rh− mother
hemophilia
genetic disorder characterized by inadequate synthesis of clotting factors
hemopoiesis
production of the formed elements of blood
hemopoietic growth factors
chemical signals including erythropoietin, thrombopoietin, colony-stimulating factors, and interleukins that regulate the differentiation and proliferation of particular blood progenitor cells
hemorrhage
excessive bleeding
hemostasis
physiological process by which bleeding ceases
heparin
short-acting anticoagulant stored in mast cells and released when tissues are injured, opposes prothrombin
Hypoxic
Consisting of too little oxygen
immunoglobulins
(also, antibodies or gamma globulins) antigen-specific proteins produced by specialized B lymphocytes that protect the body by binding to foreign objects such as bacteria and viruses
interleukins
signaling molecules that may function in hemopoiesis, inflammation, and specific immune responses
intrinsic pathway
initial coagulation pathway that begins with vascular damage or contact with foreign substances, and results in the activation of the common pathway
jaundice
yellowing of the skin or whites of the eyes due to excess bilirubin in the blood
leukemia
cancer involving leukocytes
leukocyte
(also, white blood cell) colorless, nucleated blood cell, the chief function of which is to protect the body from disease
leukocytosis
excessive leukocyte proliferation
leukopenia
below-normal production of leukocytes
lymphocytes
agranular leukocytes of the lymphoid stem cell line, many of which function in specific immunity
lymphoid stem cells
type of hematopoietic stem cells that gives rise to lymphocytes, including various T cells, B cells, and NK cells, all of which function in immunity
lysozyme
digestive enzyme with bactericidal properties
macrophage
phagocytic cell of the myeloid lineage; a matured monocyte
megakaryocyte
bone marrow cell that produces platelets
memory cell
type of B or T lymphocyte that forms after exposure to a pathogen
monocytes
agranular leukocytes of the myeloid stem cell line that circulate in the bloodstream; tissue monocytes are macrophages
myeloid stem cells
type of hematopoietic stem cell that gives rise to some formed elements, including erythrocytes, megakaryocytes that produce platelets, and a myeloblast lineage that gives rise to monocytes and three forms of granular leukocytes (neutrophils, eosinophils, and basophils)
natural killer (NK) cells
cytotoxic lymphocytes capable of recognizing cells that do not express “self” proteins on their plasma membrane or that contain foreign or abnormal markers; provide generalized, nonspecific immunity
neutrophils
granulocytes that stain with a neutral dye and are the most numerous of the leukocytes; especially active against bacteria
plasma
in blood, the liquid extracellular matrix composed mostly of water that circulates the formed elements and dissolved materials throughout the cardiovascular system
plasmin
blood protein active in fibrinolysis
platelet plug
accumulation and adhesion of platelets at the site of blood vessel injury
platelets
(also, thrombocytes) one of the formed elements of blood that consists of cell fragments broken off from megakaryocytes
polycythemia
elevated level of hemoglobin, whether adaptive or pathological
polymorphonuclear
having a lobed nucleus, as seen in some leukocytes
positive chemotaxis
process in which a cell is attracted to move in the direction of chemical stimuli
red blood cells (RBCs)
(also, erythrocytes) one of the formed elements of blood that transports oxygen
reticulocyte
immature erythrocyte that may still contain fragments of organelles
Rh blood group
blood-type classification based on the presence or absence of the antigen Rh on the erythrocyte membrane surface
Rouleaux
Collection of red blood cells resembling stacked plates
serum
blood plasma that does not contain clotting factors
sickle cell anemia
(also, sickle cell disease) inherited blood disorder in which hemoglobin molecules are malformed, leading to the breakdown of RBCs that take on a characteristic sickle shape
Stercobilins
Stercobilin is a tetrapyrrolic bile pigment and is one end-product of heme catabolism.
T lymphocytes
(also, T cells) lymphocytes that provide cellular-level immunity by physically attacking foreign or diseased cells
thalassemia
inherited blood disorder in which maturation of RBCs does not proceed normally, leading to abnormal formation of hemoglobin and the destruction of RBCs
thrombin
enzyme essential for the final steps in formation of a fibrin clot
thrombocytes
platelets, one of the formed elements of blood that consists of cell fragments broken off from megakaryocytes
thrombocytopenia
condition in which there are too few platelets, resulting in abnormal bleeding (hemophilia)
thrombocytosis
condition in which there are too many platelets, resulting in abnormal clotting (thrombosis)
thrombopoietin
hormone secreted by the liver and kidneys that prompts the development of megakaryocytes into thrombocytes (platelets)
thrombus
aggregation of fibrin, platelets, and erythrocytes in an intact artery or vein
tissue factor
protein thromboplastin, which initiates the extrinsic pathway when released in response to tissue damage
transferrin
plasma protein that binds reversibly to iron and distributes it throughout the body
universal donor
individual with type O− blood
universal recipient
individual with type AB+ blood
Urobilins
is the chemical primarily responsible for the yellow color of urine. It is a linear tetrapyrrole compound that, along with the related colorless compound urobilinogen, are degradation products of the cyclic tetrapyrrole heme.
vascular spasm
initial step in hemostasis, in which the smooth muscle in the walls of the ruptured or damaged blood vessel contracts
white blood cells (WBCs)
(also, leukocytes) one of the formed elements of blood that provides defense against disease agents and foreign materials
whole blood
blood drawn directly from the body from which none of the components, such as plasma or platelets, has been removed
Media Attributions
- olelo_noeau
- Wiki Red_White_Blood_cells © Electron Microscopy Facility at The National Cancer Institute at Frederick (NCI-Frederick) is licensed under a Public Domain license
- OS Composition of Blood © OpenStax is licensed under a CC BY (Attribution) license
- LL Chart Blood % © LynleyShimat Lys is licensed under a CC BY (Attribution) license
- OS Major Blood Components
- Hematopoietic System of Bone Marrow © OpenStax is licensed under a CC BY (Attribution) license
- Shape of Red Blood Cells © OpenStax is licensed under a CC BY (Attribution) license
- Structure of Hemoglobin © OpenStax is licensed under a CC BY (Attribution) license
- Sickle Cells © OpenStax is licensed under a CC BY (Attribution) license
- Wiki 1024px-Erythropoietin_control_of_erythrocyte_number © David Nascari and Alan Sved is licensed under a CC BY-SA (Attribution ShareAlike) license
- Erythrocyte Lifecycle © OpenStax is licensed under a CC BY (Attribution) license
- Granular Leukocytes © OpenStax Anatomy and Physiology is licensed under a CC BY (Attribution) license
- Agranulocytes (lymphocytes and monocytes) © OpenStax adapted by LynleyShimat Lys (Cropped) is licensed under a CC BY (Attribution) license
- White Blood Cells © BruceBlaus is licensed under a CC BY (Attribution) license
- Basophil © Dr Erhabor Osaro is licensed under a CC BY-SA (Attribution ShareAlike) license
- Eosinophil © Davidcsaba Dr. David Csaba L. adapted by LynleyShimat (Cropped) is licensed under a Public Domain license
- Neutrophil © Davidcsaba Dr. David Csaba L. adapted by LynleyShimat Lys (Cropped) is licensed under a Public Domain license
- Monocyte © Animalculist adapted by LynleyShimat Lys (Cropped) is licensed under a CC BY-SA (Attribution ShareAlike) license
- Lymphocyte © Guy Waterval adapted by Apache License is licensed under a CC BY (Attribution) license
- Emigration © OpenStax is licensed under a CC BY (Attribution) license
- Summary of Formed Elements in Blood © OpenStax adapted by LynleyShimat Lys (reformat to 1mb size) is licensed under a CC BY (Attribution) license
- Platelets © OpenStax is licensed under a CC BY (Attribution) license
- Hemostasis © OpenStax adapted by LynleyShimat Lys (Reformatted to 1mb size) is licensed under a CC BY (Attribution) license
- ABO Blood Group © OpenStax is licensed under a CC BY (Attribution) license
- Cross Matching Blood Types © OpenStax is licensed under a CC BY (Attribution) license
- Blood Typing © Bailliere, Tindall and Cox is licensed under a CC BY (Attribution) license
- Hemolytic Disease of the Newborn © Open Stax and Linda Bruslind is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Honu_‘Iwalani Clayton_CCBY_2022 10 30 © ‘Iwalani Clayton is licensed under a CC BY (Attribution) license
- divider_maile
(also, red blood cell) mature myeloid blood cell that is composed mostly of hemoglobin and functions primarily in the transportation of oxygen and carbon dioxide
(also, white blood cell) colorless, nucleated blood cell, the chief function of which is to protect the body from disease
blood-type classification based on the presence or absence of the antigen Rh on the erythrocyte membrane surface
liquid connective tissue composed of formed elements—erythrocytes, leukocytes, and platelets—and a fluid extracellular matrix called plasma; component of the cardiovascular system
(also, thrombocytes) one of the formed elements of blood that consists of cell fragments broken off from megakaryocytes
in blood, the liquid extracellular matrix composed mostly of water that circulates the formed elements and dissolved materials throughout the cardiovascular system
oxygen-carrying compound in erythrocytes
(also, packed cell volume) volume percentage of erythrocytes in a sample of centrifuged blood
blood drawn directly from the body from which none of the components, such as plasma or platelets, has been removed
thin, pale layer of leukocytes and platelets that separates the erythrocytes from the plasma in a sample of centrifuged blood
protein hormone secreted in response to low oxygen levels that triggers the bone marrow to produce red blood cells
deficiency of red blood cells or hemoglobin
elevated level of hemoglobin, whether adaptive or pathological
created by plasma proteins, namely albumin, that do not diffuse readily across the capillary membrane and serves to hold water within the vascular space
most abundant plasma protein, accounting for most of the osmotic pressure of plasma
heterogeneous group of plasma proteins that includes transport proteins, clotting factors, immune proteins, and others
(also, immunoglobulins or gamma globulins) antigen-specific proteins produced by specialized B lymphocytes that protect the body by binding to foreign objects such as bacteria and viruses
(also, antibodies or gamma globulins) antigen-specific proteins produced by specialized B lymphocytes that protect the body by binding to foreign objects such as bacteria and viruses
plasma protein produced in the liver and involved in blood clotting
cellular components of blood; that is, erythrocytes, leukocytes, and platelets
type of B or T lymphocyte that forms after exposure to a pathogen
production of the formed elements of blood
class of proteins that act as autocrine or paracrine signaling molecules; in the cardiovascular system, they stimulate the proliferation of progenitor cells and help to stimulate both nonspecific and specific resistance to disease
hematopoietic stem cell that gives rise to the formed elements of blood
chemical signals including erythropoietin, thrombopoietin, colony-stimulating factors, and interleukins that regulate the differentiation and proliferation of particular blood progenitor cells
type of pluripotent stem cell that gives rise to the formed elements of blood (hemocytoblast)
type of hematopoietic stem cell that gives rise to some formed elements, including erythrocytes, megakaryocytes that produce platelets, and a myeloblast lineage that gives rise to monocytes and three forms of granular leukocytes (neutrophils, eosinophils, and basophils)
bone marrow cell that produces platelets
agranular leukocytes of the myeloid stem cell line that circulate in the bloodstream; tissue monocytes are macrophages
leukocytes with abundant granules in their cytoplasm; specifically, neutrophils, eosinophils, and basophils
granulocytes that stain with a neutral dye and are the most numerous of the leukocytes; especially active against bacteria
granulocytes that stain with eosin; they release antihistamines and are especially active against parasitic worms
granulocytes that stain with a basic (alkaline) stain and store histamine and heparin
type of hematopoietic stem cells that gives rise to lymphocytes, including various T cells, B cells, and NK cells, all of which function in immunity
agranular leukocytes of the lymphoid stem cell line, many of which function in specific immunity
cytotoxic lymphocytes capable of recognizing cells that do not express “self” proteins on their plasma membrane or that contain foreign or abnormal markers; provide generalized, nonspecific immunity
hormone produced by the liver that stimulates platelet production
signaling molecules that may function in hemopoiesis, inflammation, and specific immune responses
diagnostic test of a sample of red bone marrow
treatment in which a donor’s healthy bone marrow with its stem cells replaces diseased or damaged bone marrow of a patient
inherited blood disorder in which maturation of RBCs does not proceed normally, leading to abnormal formation of hemoglobin and the destruction of RBCs
(also, sickle cell disease) inherited blood disorder in which hemoglobin molecules are malformed, leading to the breakdown of RBCs that take on a characteristic sickle shape
cancer involving leukocytes
glycoproteins that trigger the proliferation and differentiation of myeloblasts into granular leukocytes (basophils, neutrophils, and eosinophils)
nucleated cell occurring in red marrow as a stage or stages in the development of the red blood cell, or erythrocyte
immature erythrocyte that may still contain fragments of organelles
collection of red blood cells resembling stacked plates
heme-containing globular protein that is a constituent of hemoglobin
red, iron-containing pigment to which oxygen binds in hemoglobin
Consisting of too little oxygen
(also, erythrocytes) one of the formed elements of blood that transports oxygen
destruction (lysis) of erythrocytes and the release of their hemoglobin into circulation
phagocytic cell of the myeloid lineage; a matured monocyte
plasma protein that binds reversibly to iron and distributes it throughout the body
green bile pigment produced when the non-iron portion of heme is degraded into a waste product; converted to bilirubin in the liver
yellowish bile pigment produced when iron is removed from heme and is further broken down into waste products
localized bleeding under the skin due to damaged blood vessels
is the chemical primarily responsible for the yellow color of urine. It is a linear tetrapyrrole compound that, along with the related colorless compound urobilinogen, are degradation products of the cyclic tetrapyrrole heme
Stercobilin is a tetrapyrrolic bile pigment and is one end-product of heme catabolism.
yellowing of the skin or whites of the eyes due to excess bilirubin in the blood
a chronic disease of the liver marked by degeneration of cells, inflammation, and fibrous thickening of tissue. It is typically a result of alcoholism or hepatitis
(also, leukocytes) one of the formed elements of blood that provides defense against disease agents and foreign materials
process in which a cell is attracted to move in the direction of chemical stimuli
(also, emigration) process by which leukocytes squeeze through adjacent cells in a blood vessel wall to enter tissues
having a lobed nucleus, as seen in some leukocytes
digestive enzyme with bactericidal properties
antimicrobial proteins released from neutrophils and macrophages that create openings in the plasma membranes to kill cells
short-acting anticoagulant stored in mast cells and released when tissues are injured, opposes prothrombin
leukocytes with few granules in their cytoplasm; specifically, monocytes, lymphocytes, and NK cells
(also, T cells) lymphocytes that provide cellular-level immunity by physically attacking foreign or diseased cells
(also, B cells) lymphocytes that defend the body against specific pathogens and thereby provide specific immunity
below-normal production of leukocytes
excessive leukocyte proliferation
platelets, one of the formed elements of blood that consists of cell fragments broken off from megakaryocytes
condition in which there are too many platelets, resulting in abnormal clotting (thrombosis)
condition in which there are too few platelets, resulting in abnormal bleeding (hemophilia)
physiological process by which bleeding ceases
initial step in hemostasis, in which the smooth muscle in the walls of the ruptured or damaged blood vessel contracts
accumulation and adhesion of platelets at the site of blood vessel injury
formation of a blood clot; part of the process of hemostasis
insoluble, filamentous protein that forms the structure of a blood clot
group of 12 identified substances active in coagulation
genetic disorder characterized by inadequate synthesis of clotting factors
initial coagulation pathway that begins with vascular damage or contact with foreign substances, and results in the activation of the common pathway
initial coagulation pathway that begins with tissue damage and results in the activation of the common pathway
final coagulation pathway activated either by the intrinsic or the extrinsic pathway, and ending in the formation of a blood clot
protein thromboplastin, which initiates the extrinsic pathway when released in response to tissue damage
enzyme essential for the final steps in formation of a fibrin clot
aggregation of fibrin, platelets, and erythrocytes in an intact artery or vein
gradual degradation of a blood clot
blood protein active in fibrinolysis
thrombus that has broken free from the blood vessel wall and entered the circulation
blood-type classification based on the presence or absence of A and B glycoproteins on the erythrocyte membrane surface
clustering of cells into masses linked by antibodies
excessive bleeding
individual with type O− blood
individual with type AB+ blood
blood test for identification of blood type using antibodies and small samples of blood
blood plasma that does not contain clotting factors
(also, erythroblastosis fetalis) disorder causing agglutination and hemolysis in an Rh+ fetus or newborn of an Rh− mother
