Anatomy and Physiology
22 Immunology
I paʻa ke kino o ke keiki i ka lāʻau.
That the body of the child be solidly built by the medicines.
A mother ate herbs during pregnancy and nursing for the sake of the baby’s health. The herbs were given to the child up to the age of twenty so that he would be healthy and strong through maturity and old age.
ʻŌlelo Noʻeau, compiled by Mary Kawena Pukui, #1252
Introduction

Figure 22.1: Kealakekua Bay and the Village Kowroaa: Kaʻawaloa in 1779 by John Webber, artist aboard Cook’s ship
The function of the immune system is to resist development. The immune system works by recognizing (binding) and destroying molecules that are foreign (different from your own). Sometimes your own body cells become damaged and are recognized as abnormal and destroyed. In the case of autoimmune disease, components of your immune system bind and attack healthy tissues. Generally speaking, the immune system slowly evolves to resist diseases that are in the surrounding environment. It may be unprepared, however, to deal with new foreign threats. When geographically isolated peoples come into contact, such as when Captain Cook and his crew first arrived in the Hawaiian Islands, the immune systems of each respective group often aren’t able to resist the s carried by the other. In such situations, diseases may spread quickly, often with devastating consequences.
![]() Chapter Learning Outcomes
Chapter Learning Outcomes
- Describe the various types of pathogens
- Detail how our bodies recognize foreign cells and molecules
- Distinguish between the innate and adaptive immune systems
- Relate specific diseases to pathogens, symptoms, and the immune response
22.1 Types of Pathogens
![]() 22.1 Learning Outcomes
22.1 Learning Outcomes
- Compare and contrast the various types of pathogens
- Relate normal flora to homeostasis
- Explain how leukocytes recognize and prevent disease

Figure 22.2: Relative Size of Viruses and Bacteria (OpenStax Microbiology)
Pathogens are biological agents that cause disease (abnormal tissue/ organ function). There are many different types of pathogens. Some pathogens, such as , are prokaryotic and structurally very different from your eukaryotic cells. These are easier to be recognized by your immune system as a foreign threat. Other pathogens may be eukaryotic, appearing more similar to your own cells and generally harder to recognize as foreign. The greater the molecular differences between your cells and a pathogen, the more likely the immune system will be able to bind and destroy it. Size can make a difference too, as larger molecules are more likely to be recognized than smaller ones.
Bacteria are living, prokaryotic microbes usually with lipopolysaccharide or peptidoglycan components in the cell walls. These large molecules have structural patterns absent from human cells and therefore are usually easily recognized by the immune system. If not stopped, some bacteria may adhere to the body and multiply using your body as a food source. Many bacteria also produce and secrete toxins that may contribute to disease symptoms.
![]() Cultural Connection
Cultural Connection
Hansen’s Disease
Geographical isolation allows diseases to spread quickly once introduced. Since the arrival of Captain Cook, epidemics introduced by explorers such as mumps, smallpox, measles, influenza and dysentery have decimated the population of the Hawaiian Islands which contributed to the subsequent colonization. One such disease was , formerly known as leprosy. In 1866, the Kalaupapa leprosarium was established on the Hawaiian island of Molokaʻi as a place to kipaku (banish) thousands of Native Hawaiians infected by the disease. Disagreement exists over the extent of the loss of life, with some estimates indicating that between 30-80% of Native Hawaiians perished due to the leprosy epidemic.

Figure 22.3: The Bishop Home for Boys, part of the Kalauau leprosarium, with inset picture of children with Hansen’s disease playing along the coral reefs on the shore of Molokaʻi
Hansen’s disease is caused by a slow-growing bacteria called Mycobacterium leprae. Once inside the body, M. leprae can affect the nerves, skin, eyes, and lining of the nose (nasal mucosa). Signs and symptoms of Hansen’s disease are often most noticeable on the skin and include discolored lesions, growths, thick or dry patches, painless ulcers on the bottoms of the feet, painless lumps on the face, or ears, and loss of facial hair such as eyebrows and eyelashes. Individuals with long-term Hansen’s disease have noticeable facial disfigurements. Neurologically, numbness and (pins and needles) are common, and sufferers often experience muscle weakness or paralysis. Damage to nerves in the face and eyes can also lead to blindness. M. leprae has a low and uncertainty exists on the exact mechanism of how Hansen’s disease is spread from person to person.

Figure 22.4: (a) The nose of a patient with Hansen’s disease. Note the lepromatous/multibacillary lesions around the nostril. (b) Hansen’s disease is caused by Mycobacterium leprae, a gram-positive bacillus. (credit a, b: modifications of work by the Centers for Disease Control and Prevention) (OpenStax Microbiology)
es are non-living but can still be pathogens. Due to their implications for health and disease, virology is often covered in introductory microbiology courses. Viruses are simply relatively small strands of DNA or RNA wrapped in protein. Because viruses are non-living, they require living cells to propagate, which often causes pathology in the process. Even though they are much smaller than bacteria and replicate inside living cells, viruses have unique molecular (protein, DNA, or RNA) patterns that our immune system is able to recognize as foreign.
are eukaryotic organisms and some species are pathogens. Even though they consist of eukaryotic cells, fungi often contain cell wall components such as chitin and glucans that are dissimilar to any human molecule, thus allowing for recognition.
![]() Local Issue
Local Issue
Tinea versicolor (aka haole rot, pityriasis versicolor) is a pathology caused by fungus species in the Malassezia genus. This fungus feeds off lipids and dead cells on the skin surface causing discoloration and superficial scaling. In Hawaiʻi, the term haole (foreigner) is sometimes used to describe a lighter-skinned individual. The white spots caused by this fungus can make it look like tan skin has lighter patches thus the inspiration for the name haole rot commonly used by locals in Hawaiʻi. It is important to note, that though commonly used in the past, using the word “haole” in casual conversation is becoming politically incorrect. Tinea versicolor is more common in hot humid environments and in people with hyperhidrosis (excessive perspiration).

Figure 22.5: Tinea versicolor, also known as Haole rot, pityriasis versicolor
Protists and worms are other examples of potential eukaryotic human pathogens. Protists are a group of single celled microorganisms whereas worms are multicellular. Both include species known to be parasitic pathogens. The diverse prokaryotic domain archaea contains no known human pathogens, nor does the multicellular eukaryotic plant kingdom aside from potential toxicity. Viroids, a virus-like nucleic acid but without a protein coat, are only known to cause disease in plants. Whether a living microbe or not, all the above contain nucleic acids (DNA and/or RNA). s are proteins capable of causing disease directly. Proteins have a specific three dimensional shape that determines its function. Prion proteins take on an altered shape and, upon contact, cause normally shaped proteins to do the same. These misfolded proteins build up in cells causing disease.
Not all microbes are pathogenic! Your skin and mucous membranes are covered in microbes that don’t cause disease. The include bacteria, fungi, viruses among others and prevent disease by outcompeting pathogens for nutrients. Normal flora can vary between people with different diets, lifestyles and living environments. In addition, different areas of the body house different microbe populations. Using normal flora microbes as a method for treating disease is a growing area of medical research.
How does the immune system “recognize” foreign molecules? As we previously learned in the hematology chapter, cells of the immune system are called s (leuko = white, cyte = cells, aka white blood cells, WBCs). Leukocytes have receptors that bind foreign molecules triggering the release of (AMPs), digestive enzymes and/or s. Cytokines stimulate other cells to facilitate and amplify the immune response. Just like RBCs, all WBCs are produced in the bone marrow. From there, leukocytes may travel to reside in the peripheral tissues or circulate the body via the cardiovascular and s.
22.2 Lymphatic System
![]() 22.2 Learning Outcomes
22.2 Learning Outcomes
- List the general functions of the lymphatic system
- Describe the process of lymph return to the cardiovascular system
- Label and describe general lymph node structure and function
- List the specialized lymphoid tissues associated with the GI tract
The function of the lymphatic system is to concentrate and screen for pathogens in areas of your body most likely to get infected. Plasma that leaves the cardiovascular system and enters the tissues becomes interstitial fluid (IF, inter = between, stitial = tissues). Most of that fluid returns to the cardiovascular system at the venous end of capillary beds. However, about 20% of the remaining IF enters as (fluid of the lymphatic system).

Figure 22.6: Lymphatic Capillaries Lymphatic capillaries are interlaced with the arterioles and venules of the cardiovascular system. Collagen fibers anchor a lymphatic capillary in the tissue (inset). Interstitial fluid slips through spaces between the overlapping endothelial cells that compose the lymphatic capillary.
 |
 |
| Precapillary Sphincters and Valves: (a) Precapillary sphincters are rings of smooth muscle that regulate the flow of blood through capillaries; they help control the location of blood flow to where it is needed. (b) Valves in the veins prevent blood from moving backward. (credit a: modification of work by NCI) (OpenStax Biology 2e) | Lymph Capillaries in the Tissue Spaces: Fluid from the capillaries moves into the interstitial space and lymph capillaries by diffusion down a pressure gradient and also by osmosis. Out of 7,200 liters of fluid pumped by the average heart in a day, over 1,500 liters is filtered. (credit: modification of work by NCI, NIH) (OpenStax Biology 2e) |

|
|
| Capillary Exchange Net filtration occurs near the arterial end of the capillary since capillary hydrostatic pressure (CHP) is greater than blood colloidal osmotic pressure (BCOP). There is no net movement of fluid near the midpoint since CHP = BCOP. Net reabsorption occurs near the venous end since BCOP is greater than CHP. | |
Figure 22.7: Lymph Capillaries and Pressure gradient
Lymphatic capillaries are low-pressure single-cell thick vessels that avoid collapsing by . Lymph enters , slits in between adjacent cells. Similar to how blood moves in veins, lymph moves in the direction of the heart due to a pressure gradient difference caused by the and the prevention of backflow by lymphatic . In addition, pulsatile movement of blood in adjacent arteries and smooth muscles of larger prevents lymph stagnation. Lymphatic capillaries combine to form the larger lymphatic vessels. Lymphatic vessels deliver lymph to s.
 |
|
| Location, Structure, and Histology of the Thymus The thymus lies above the heart. The trabeculae and lobules, including the darkly staining cortex and the lighter staining medulla of each lobule, are clearly visible in the light micrograph of the thymus of a newborn. LM × 100. (Micrograph provided by the Regents of the University of Michigan Medical School © 2012) | |
 |
|
| Major Trunks and Ducts of the Lymphatic System The thoracic duct drains a much larger portion of the body than does the right lymphatic duct. | |
 |
 |
| Structure and Histology of a Lymph Node Lymph nodes are masses of lymphatic tissue located along the larger lymph vessels. The micrograph of the lymph nodes shows a germinal center, which consists of rapidly dividing B cells surrounded by a layer of T cells and other accessory cells. LM × 128. (Micrograph provided by the Regents of the University of Michigan Medical School © 2012) | Mucosa-associated Lymphoid Tissue (MALT) Nodule LM × 40. (Micrograph provided by the Regents of the University of Michigan Medical School © 2012) |
Figure 22.8: Lymph Nodes, Lymphatic Trunks and Ducts
Lymph nodes house many leukocytes that bind foreign particles present in the lymph and initiate an immune response. The node is divided into medullary and cortical regions contained by an outer fibrous capsule which extends inward forming trabeculae. These divide the cortex into several lobules. Lymph enters lymph nodes through on the convex side and exits via at the hilum.

Figure 22.9: Lymphatic System Trunks: Terminal collecting trunks of right side. a. Jugular trunk. b. Subclavian trunk. c. Bronchomediastinal trunk. d. Right lymphatic trunk. e. Gland of internal mammary chain. f. Gland of deep cervical chain.
As lymph moves toward the heart, lymphatic vessels combine to make bigger , and trunks eventually fuse to form the larger lymphatic ducts. There are only two lymphatic ducts. The returns lymph from the upper right quadrant of the body to the cardiovascular system at the junction of the right subclavian and right internal jugular veins. The returns lymph from the rest of the body at the left subclavian — left jugular vein junction.

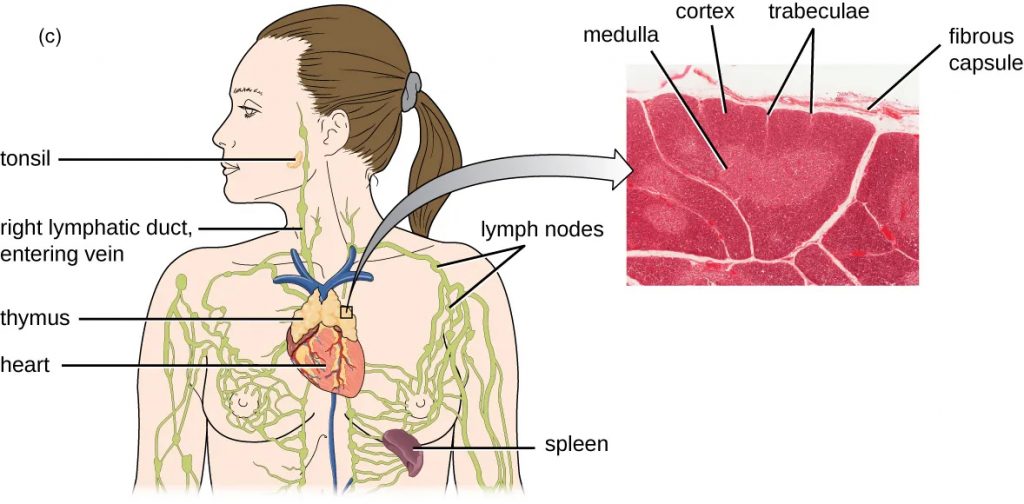
Figure 22.10 (a) Red bone marrow can be found in the head of the femur (thighbone) and is also present in the flat bones of the body, such as the ilium and the scapula. (b) Red bone marrow is the site of production and differentiation of many formed elements of blood, including leukocytes. (c) The is a bi-lobed, H-shaped glandular organ that is located just above the heart. It is surrounded by a fibrous capsule of connective tissue. The darkly staining cortex and the lighter staining medulla of individual lobules are clearly visible in the light micrograph.
The bone marrow and the thymus are considered the s because they are involved in leukocyte development. s (lymph nodes) house mature leukocytes to facilitate activation of the immune system. Lymph nodes are found in areas of the body that are at high risk for invading bacteria such as the axillary, inguinal, and cervical areas.

Figure 22.11: The spleen is similar to a lymph node but is much larger and filters blood instead of lymph. Blood enters the spleen through arteries and exits through veins. The spleen contains two types of tissue: and . Red pulp consists of cavities that store blood. Within the red pulp, damaged red blood cells are removed and replaced by new ones. White pulp is rich in that remove -coated bacteria from the blood.
Though the spleen is primarily responsible for filtering blood, not lymph, it is often considered the body’s largest lymph node. It has a similar organization as other lymph nodes. Between trabeculae, red pulp areas are where damaged RBCs are broken down and white pulp areas contain leukocytes performing more typical lymph node functions.

Figure 22.12: Mucosa-associated Lymphoid Tissue (MALT) Nodule LM Å~ 40. (Micrograph provided by the Regents of the University of Michigan Medical School ˝ 2012)
Other specialized lymph nodes are associated with the oral cavity () and the digestive system ( and = mucosa-associated lymphoid tissue). Part of the MALT is the which is the lymphoid nodules associated with the respiratory tract. s are the specialized lymphatic capillaries associated with the gut. Lacteals absorb bile and lipids from the digestive tract. This fluid is called (lymph and chyme) and has a milky appearance quite distinct from regular lymph. It drains into the (the swollen inferior end of the thoracic duct).
22.3 Innate Immunity
![]() 22.3 Learning Outcomes
22.3 Learning Outcomes
- List the physical and chemical barriers preventing pathogen infection
- Compare and contrast pathogen recognition by cells of innate immunity
- Detail the role of inflammation including complement and fever
- Describe the process of WBC recruitment to a site of infection
refers to the anatomical structures and physiological processes in place at birth that provide resistance to pathogens entering, binding, and causing disease to the body. These innate mechanisms of disease resistance can be divided into the first and second lines of defense.

Figure 22.13: Innate Immune System Table: The innate immune system is made up of physical barriers and internal defenses.

Figure 22.14: Phagocytic cells contain s (PRRs) capable of recognizing various pathogen-associated molecular patterns (). These PRRs can be found on the plasma membrane or in internal phagosomes. When a PRR recognizes a PAMP, it sends a signal to the nucleus that activates genes involved in , cellular proliferation, production and secretion of antiviral interferons and proinflammatory cytokines, and enhanced intracellular killing.
The first line of defense includes barriers such as your skin and mucous membranes that are physically difficult to cross. These boundaries contain chemical substances that resist pathogen adherence and propagation. For instance, sweat, tears, and saliva are acidic and contain antimicrobial peptides. The first line of defense barriers may become compromised due to injury or other conditions. In these cases, the second line of defense mechanisms comes into play. The cells of innate immunity contain receptors that bind certain patterns common among pathogens but are drastically different from those found on human cells. PAMPs (pathogen-associated molecular patterns) are those molecular patterns on pathogens your innate immune system can recognize. Your tissues that have become damaged through injury may also elicit molecular patterns (, damage-associated molecular patterns) that your innate immune cell receptors can bind. In either case, the receptors on innate immune cells that bind PAMPs and DAMPs are called .
PAMPs include the bacterial macromolecules lipopolysaccharide (LPS) and peptidoglycan. Some viruses and bacteria contain unique DNA and RNA sequences that can be recognized by PRRs. Fungal pathogens can be recognized by PRRs specific for the PAMPs on glucan and chitin. Innate immune cells with PRRs can be divided into two major classes: the (cells with granules) and (cells without granules).
Cells of Innate Immunity

Figure 22.15: Granular Leukocytes. A has small granules that stain light lilac and a nucleus with two to five lobes. An ’s granules are slightly larger and stain reddish-orange, and its nucleus has two to three lobes. A has large granules that stain dark blue to purple and a two-lobed nucleus.

Figure 22.16: An , such as a , engulfs and digests a foreign bacterium. An antigen from the bacterium is presented on the cell surface in conjunction with an MHC molecule. Lymphocytes of the adaptive immune response become activated when they bind the MHC embedded foreign molecules.
Since they have multilobed nuclei of various shapes, granulocytes are also known as polymorphonuclear cells (s). There are three types of granulocytes. Neutrophils are the most abundant of all the leukocytes. They are also typically the first responders to a site of infection. When their PRRs bind a PAMP, the membrane can wrap around and engulf the pathogen in a process known as phagocytosis. Once the pathogen is internalized, it is broken down by digestive enzymes released from cytoplasmic granules. Neutrophils may also release chromatin forming neutrophil extracellular traps () that help prevent the spread of pathogens. Neutrophils are destroyed in the process, therefore these cells have a high turnover rate, typically lasting only 10 days or so. Eosinophils have PRRs and granule enzymes for attacking certain parasites. Basophils (and the similar s) reside in the peripheral tissues of adjacent mucous membranes and contain granules with and other inflammatory molecules. Both eosinophils and basophils are among the least abundant leukocytes and often implicated in allergic reactions.

Figure 22.17: Natural killer (NK) cells are inhibited by the presence of the major histocompatibility cell (MHC) receptor on healthy cells. Cancer cells and virus-infected cells have reduced expression of MHC and increased expression of activating molecules. When a NK cell recognizes decreased MHC and increased activating molecules, it will kill the abnormal cell.
Agranular cells of innate immunity include macrophages (macro = big, phage = to eat), dendritic and s (NK). Originating as blood s, macrophages and s are s like neutrophils. However, macrophages and dendritic cells activate cells of the adaptive immune system in a process called . Therefore, these cells are known as APCs (antigen presentation cells) and act as a bridge to the body’s more evolutionary advanced adaptive immune defenses. Part of the lymphocyte lineage, NK cells are primarily responsible for monitoring your own cells. NK cells release s and s to destroy damaged or irregular cells. Perforins, make a hole (perforate) in cell membranes and granzymes are proteases that induce target cell apoptosis.

Figure 22.18: Innate immune system cells. The characteristics and location of cells involved in the innate immune system are described. (credit: modification of work by NIH)
Inflammation

Figure 22.19: Acute Inflammation: Resident leukocytes and damaged cells release histamine and other inflammatory chemicals in response to infection or injury. The increased blood flow brings fluid, proteins, phagocytes, and other immune cells to enter infected tissue. These events result in swelling, reddening and pain around the injured site.
What is the innate immune response to a puncture wound, such as a cut with a dirty knife? Your first line of defense, physical and chemical barriers, have been compromised and now you must rely on the second line of defense mechanisms to stop pathogens from growing in your body. How are cells of the innate immune system called to the site of injury? What damage control mechanisms occur to allow the body time to heal? The answer to both these questions is ; one of the hallmarks of innate immunity. If you’ve ever experienced acute inflammation before, you may easily recognize the cardinal signs: redness, heat, swelling, and ʻeha (pain). Some consider loss of function or impaired function to be the fifth cardinal sign. Words ending with the suffix -itis are used to describe inflammation of specific organs and tissues. For example, dermatitis refers to inflammation of the skin (dermis) and pericarditis refers to inflammation of the pericardium around the heart.
The four phases of inflammation are:
- Tissue injury causing inflammatory chemical release
- Vasodilation
- Increased vascular permeability
- Phagocyte mobilization
It is important to note that inflammation in response to tissue injury and/or infection is the first step towards healing. Benefits of the localized acute inflammatory response include:
- Impeding the spread of pathogens
- Destroying pathogens
- Removing debris and damaged tissue
- Repairing tissue
- Activating additional immune defenses

Figure 22.20: Interferons are cytokines released by a cell infected with a virus. Interferon-α and interferon-β signal uninfected neighboring cells to inhibit mRNA synthesis, destroy RNA, and reduce protein synthesis (top arrow). Interferon-α and interferon-β also promote apoptosis in cells infected with the virus (middle arrow). Interferon-γ alerts neighboring immune cells to an attack (bottom arrow). Although interferons do not cure the cell releasing them or other infected cells, which will soon die, their release may prevent additional cells from becoming infected, thus stemming the infection.
| Cytokines | Stimulate and modulate most functions of immune system | |
| Chemokines | Recruit white blood cells to infected area | |
| Interferons | Alert cells to viral infection, induce apoptosis of virus-infected cells, induce antiviral defenses in infected and nearby uninfected cells, stimulate immune cells to attack virus-infected cells |
Table 22.1: Chemical Defenses of Nonspecific Innate Immunity – Cytokines (OpenStax Microbiology)
Inflammation can be triggered by several factors including physical trauma, intense heat, irritating chemicals, and infection by a pathogen. As a result of tissue injury, inflammatory chemicals are released into the extracellular fluid to alert the rest of the body that there is a problem and healing is needed. Some of these are cytokines that alert the rest of the immune system, via both short (autocrine and paracrine) and long-distance (endocrine) communication. Common cytokines involved in the inflammatory response include (colony-stimulating factors), s, interferons, and interleukins. CSFs induce (leukocyte proliferation in bone marrow), chemokines act as a homing beacon for the site of injury, interferons impede viral production within cells, and interleukins allow one WBC to stimulate others (inter = between, leuk = leukocytes, in = protein). are released by virus-infected cells and bind receptors of other cells, causing them to be more resistant to viral infection. Typically, INFs interfere with viral nucleic acid replication in some way. There are many different types of interferons, INF𝞪, INF𝛃, and INF𝞬 being the most well-known. The INFs secreted by infected cells can also activate other immune cells such as NK cells and macrophages.
| Inflammation-eliciting mediators | Histamine | Promotes vasodilation, bronchoconstriction, smooth muscle contraction, increased secretion and mucus production |
| Leukotrienes | Promote inflammation; stronger and longer lasting than histamine | |
| Prostaglandins | Promote inflammation and | |
| Bradykinin | Increases vasodilation and vascular permeability, leading to edema |
Table 22.2: Chemical Defenses of Nonspecific Innate Immunity – Inflammation-eliciting mediators (OpenStax Microbiology)
Other inflammatory chemicals exacerbate the inflammation response itself. These include histamine, , , and the family of plasma proteins. All of these contribute to (increase in blood vessel diameter) and promote cell migration to the site of injury. Vasodilation of the local arterioles results in (increased blood flow) near the site. Inflammatory mediators also increase the permeability of local vasculature, causing leakage of fluid into the interstitial space, resulting in edema (swelling) and redness. The increase in pressure causes heat and pain in the area. Pain can also be caused by the toxins secreted by invading pathogens or chemicals released by damaged tissues.
The flow of fluid to a site of infection helps to sequester (isolate, to keep separate) pathogens. Protein-rich fluids of the plasma enter the tissues and help move foreign material into the surrounding lymphatic vessels for transport to the lymph nodes. Clotting factors that facilitate wound repair also move into the area.
Complement System

Figure 22.21: The classic pathway for the complement cascade involves the attachment of several initial complement proteins to an -bound pathogen followed by rapid activation and binding of many more complement proteins and the creation of destructive pores in the microbial cell envelope and cell wall. The alternate pathway does not involve antibody activation. Rather, convertase spontaneously breaks down C3. Endogenous regulatory proteins
Complement is a family of proteins that serve to enhance both the innate and adaptive immune responses. The complement system is a cascade of events initiated by either the classical, alternative, or s. It begins with several proteins becoming activated by enzymatic fragmentation in the presence of a pathogen. In the , complement proteins are activated by the presence of antibodies (, y-shaped proteins of the adaptive immune system). The alternate pathway is initiated by a pathogen’s lack of the inhibitory proteins that are present on your cells. The lectin pathway stimulates the complement cascade when mannose residues are exposed on some bacterial cell walls. The activated fragments then activate other complement proteins which, in turn, activate others, and so on. Ultimately this process leads to destruction of bacteria, enhanced inflammation and mobilization of leukocytes. Key complement proteins include C3 and which, when activated, produce the fragments C3a, C3b, C5a and C5b. C3a and C5a both increase vascular permeability, smooth muscle construction and mast cell degranulation. C3b is part of the protease that activates C5. C5b initiates formation of the (membrane attack complex) on the surface of certain target cells by triggering the interaction of C6, C7, C8 and several C9s. The polymerization of C9 creates a hole in bacterial membranes disrupting the target cell. Additionally, C3b is an opsonin which is a molecule that enhances phagocytosis by binding to the pathogen surface. Neutrophils and macrophages have C3b receptors allowing them to recognize C3b coated pathogens more readily and initiate phagocytosis.
![]() Clinical Application
Clinical Application
The Immune Response and NSAIDs
Itchy eyes? Stuffed up nose? Pain and swelling after ankle injury? Fever due to infection? Traditionally, the average American would treat such signs and symptoms with a medicinal drug. Common non-steroidal anti-inflammatory drugs, or , such as aspirin and ibuprofen, are used on a regular basis by more than 60 million Americans to diminish the effects of inflammation. We have come to rely on these drugs, but it is important to note that they have detrimental side effects. NSAIDs reduce inflammation and fever by inhibiting the complement system and the production of prostaglandins detrimentally affecting immune system function. Aspirin and ibuprofen are also toxic to your kidney and liver, respectively.
![]() Food and Environment
Food and Environment
Nutrition and the Immune System
Have you ever heard the adage “you are what you eat”? This certainly holds true for our immune systems! Some studies have found that the immune system reacts to an unhealthy diet in much the same way it reacts to a bacterial infection or tissue injury: by initiating the inflammatory process. While some physiological inflammation is necessary and important in the healing process, chronic inflammation may damage healthy tissue and cause disease. Chronic inflammation may be caused by many things including dietary deficiencies. For instance, zinc is a micronutrient that plays an important role in many aspects of the immune system. Adequate levels of zinc allow for the normal development and functioning of many cells of the innate immune system, including neutrophils and NK cells. Zinc may also play a critical role in the development and proper functioning of the entire adaptive immune system. Thus cytokine production, phagocytosis, and the entire adaptive immune system could be negatively affected just from a zinc deficiency. Zinc is commonly found in oysters, red meat, eggs, and smaller amounts in kalo and poi!

Figure 22.22: Ahi poke made with tuna, green onions, chili peppers, sea salt, soy sauce, sesame oil, roasted kukui nut (candlenut), and limu, served on a bed of red cabbage. Kukui nuts and sesame seeds are high in zinc.
Fever
Fever, or , is an abnormally high body temperature which is part of a normal innate immune response. Pathogens and leukocytes release that signal the hypothalamus to raise body temperature. Muscle contractions increase along with the sensation of feeling cold. Secreted prostaglandins enhance the fever-inducing effect. If you’ve ever taken care of a sick keiki (child), you know a high fever can be worrisome. You may have wanted to administer (fever-reducing) medications to make them feel more comfortable. Fevers are commonly thought of as undesirable, but in fact, they benefit the body by inhibiting the production of viruses and bacteria, increasing the metabolic rate of your cells (and thus the healing process), and enhancing the actions of immune system proteins such as interferons and some enzymes. Unless it is unusually high, fevers aid in the healing process.
Antimicrobial Peptides
| Characteristics of Selected Antimicrobial Peptides (AMPs) | ||||
|---|---|---|---|---|
| AMP | Secreted by | Body site | Pathogens inhibited | Mode of action |
| Bacteriocins | Resident microbiota | Gastrointestinal tract | Bacteria | Disrupt membrane |
| Cathelicidin | Epithelial cells, macrophages, and other cell types | Skin | Bacteria and fungi | Disrupts membrane |
| Defensins | Epithelial cells, macrophages, neutrophils | Throughout the body | Fungi, bacteria, and many viruses | Disrupt membrane |
| Dermicidin | Sweat glands | Skin | Bacteria and fungi | Disrupts membrane integrity and ion channels |
| Histatins | Salivary glands | Oral cavity | Fungi | Disrupt intracellular function |
Table 22.3: Antimicrobial Peptides (AMPs) OpenStax Microbiology
There is a large variety of antimicrobial peptides (AMPs) that facilitate the innate immune response. These are chains of 12-50 amino acids that are toxic to certain microbes by disrupting cell membranes or interfering with the synthesis of nucleic acids, proteins or cell wall components. Some AMPs may block the activity of specific enzymes while others may serve as immunomodulators. In many cases, specific AMPs bind multiple targets in diverse species such as bacteria, fungi and viruses. For example, and s are two chemical classes of AMPs with antibacterial, antifungal and antiviral properties in addition to other functions. Dermcidin is a protein of over 100 amino acids that is chopped up into smaller mature peptides by sweat proteases. Defensins have propeptides that must be removed for activation which may occur within or after being secreted out of a cell. For instance, neutrophils store defensins within their granules to facilitate the digestion of phagocytized bacteria.
Phagocyte mobilization

Figure 22.23: Extravasation (Diapedesis) of Leukocytes: Damaged cells and macrophages that have ingested pathogens release cytokines that are proinflammatory and chemotactic for leukocytes. In addition, activation of complement at the site of infection results in production of the chemotactic and proinflammatory C5a. Leukocytes exit the blood vessel and follow the chemoattractant signal of cytokines and C5a to the site of infection. Granulocytes such as neutrophils release chemicals that destroy pathogens. They are also capable of phagocytosis and intracellular killing of bacterial pathogens.
As a result of tissue injury, nearby cells release interleukins, chemokines and CSFs. Some interleukins and C5a induce endothelial cells to express (cell adhesion molecules). CSFs cause (high white blood cell count). Blood cells tend to travel in the middle of vessels where friction is low. However, when CAMs are present leukocytes stick and roll along the vessel wall, slowing their velocity in a process called . During , the leukocytes stop completely and squeeze through the clefts between endothelial cells. Chemokines diffusing from the site of injury attract leukocytes via . Neutrophils are the first responders, quickly followed by macrophages. Remember, neutrophils are phagocytes specialized to destroy microbes and contain infections. Macrophages, on the other hand, are phagocytes that activate .
22.4 Adaptive Immunity
![]() 22.4 Learning Outcomes
22.4 Learning Outcomes
- Describe the role of APCs and MHC in adaptive immunity
- Illustrate the role of T helper cells and the adaptive immune response
- Distinguish between cell-mediated immunity and humoral immunity
- Compare and contrast antibody isotype structure and function
- Relate lymphocyte development and proliferation to immune function

Figure 22.24: Antigens may interact with a variety of lymphocyte receptors depending on how many binding motifs (s) are present.
Unlike cells of innate immunity, lymphocytes (T and s) each have unique, seemingly random receptors. This enables them to potentially bind any foreign object unrecognizable by PRRs of the innate immune system. Any molecule that can bind a T or B cell receptor is an antigen. Upon binding antigen, lymphocytes respond by secreting interleukins and other molecules to facilitate of the pathogen. Additionally, the lymphocytes will proliferate in a way to strengthen pathogen recognition over time and establish an . Before lymphocytes can perform any of these functions, however, the adaptive immune system must first be activated by APCs.
Major Histocompatibility Complex

Figure 22.25: Major histocompatibility complex (MHC). This figure illustrates the activation of a naive (unactivated) lymphocyte by an antigen-presenting MHC I molecule on an infected body cell. Once activated, perforins and granzymes are released inducing apoptosis in the infected cell.
The are two distinct membrane bound proteins ( and II) that play roles in antigen presentation. Most cells in your body express MHC class I. Routinely, your own proteins are digested, pieces of 8-11 amino acids bind MHC-I and are displayed on the cell surface. However, if any of these cells are infected by intracellular pathogens, pieces of digested pathogen molecules can bind MHC-I instead. Intracellular pathogens include bacteria, viruses and some multicellular parasites.

Figure 22.26: APBIO MHC-II antigen presentation by APCs.
APCs, such as macrophages and dendritic cells, express MHC-II. Remember, these cells are phagocytes. When they engulf and digest pathogens, pieces of the foreign molecules (usually 13-17 amino acids) bind MHC-II and are displayed. APCs may encounter a pathogen in the peripheral tissues or within the lymphatics. After phagocytosis, the APCs migrate to lymph nodes where s (Th)[/pb_glossary] are highly concentrated. The first and essential step in adaptive immunity activation is when an APC displays antigen to a Th with a receptor that recognizes (binds) the antigen-MHC complex. ensures any one individual the potential to display a great variety of antigens. greatly increases the variety of presented antigens within a population. However, true to its name, MHC polymorphism is the main cause of mismatched transplant rejection (major = main cause, histo = tissue, compatibility = donor/ recipient compatible).
![]() Deep Dive
Deep Dive
Love at First Smell? — MHC diversity in population and the sweaty shirt study
Wedekind, C. et al. (1995) studied the effect of MHC diversity on mating preferences in humans. Researchers recruited 49 females and 44 males for the study; males were selected specifically to ensure a variety of MHC gene types. Males were tasked with wearing the same T-shirt for two days and were not permitted to wash it or take it off, even during sleep. The males then returned the now sweaty and smelly T-shirts, and the shirts were individually packaged in boxes with a smelling hole. Females were asked to smell each box one at a time and rate the odor based on intensity, pleasantness, and sexiness. Surprisingly, it was found that females preferred the smell of the T-shirts that were worn by males whose MHC genes were different than their own. The more diverse your MHC molecules, the broader diversity of antigens you are able to present to Th cells. Perhaps the reason the women in the study preferred odors from genetically distinct males is due to an instinct to provide offspring with an advantage in recognizing pathogens.
T Cells
| Classes of T Cells | |||
|---|---|---|---|
| Class | Surface CD Molecules | Activation | Functions |
| Helper T cells | CD4 | APCs presenting antigens associated with MHC II | Orchestrate humoral and cellular immunity |
| Involved in the activation of macrophages and NK cells | |||
| Regulatory T cells | CD4 | APCs presenting antigens associated with MHC II | Involved in peripheral tolerance and prevention of autoimmune responses |
| Cytotoxic T cells | CD8 | APCs or infected nucleated cells presenting antigens associated with MHC I | Destroy cells infected with intracellular pathogens |
Table 22.4: Classes of T Cells – OpenStax Microbiology
| Subtypes of Helper T Cells | |
|---|---|
| Subtype | Functions |
| TH1 cells | Stimulate cytotoxic T cells and produce memory cytotoxic T cells |
| Stimulate macrophages and neutrophils (PMNs) for more effective intracellular killing of pathogens | |
| Stimulate NK cells to kill more effectively | |
| TH2 cells | Stimulate B cell activation and differentiation into plasma cells and memory B cells |
| Direct antibody class switching in B cells | |
| TH17 cells | Stimulate immunity to specific infections such as chronic mucocutaneous infections |
| Memory helper T cells | “Remember” a specific pathogen and mount a strong, rapid secondary response upon re-exposure |
Table 22.5: Subtypes of Helper T Cells – OpenStax Microbiology

Figure 22.27: Naïve CD4+ T cells engage MHC II molecules on antigen-presenting cells (APCs) and become activated. Clones of the activated helper T cell, in turn, activate B cells and CD8+ T cells, which become cytotoxic T cells. Cytotoxic T cells kill infected cells.
Helper T cells

Figure 22.28: Helper T Cell: This illustration depicts the activation of a naïve (unactivated) helper T cell by an antigen-presenting cell and the subsequent proliferation and differentiation of the activated T cell into different subtypes.
Th are also known as since they express the surface protein CD4. This and other outer membrane proteins are essential in the activation process. Once activated, Th cells secrete IL-2 which drives proliferation in an autocrine and paracrine manner. Depending on the cytokine environment, activated Th cells will promote adaptive immune responses to target either intracellular or extracellular pathogens by stimulating other lymphocytes to become and/or . These cells will target pathogens directly. In some cases, CD4+ T cells become that dampen the immune responses of effector cells.
Cell-mediated Immunity
To target intracellular pathogens, is activated by helper T cell subclass . This involves (aka CD8+ T cells, TC) surveying your own tissues looking for unhealthy cells. If cells in your body are cancerous or infected by a virus or other pathogen, MHC-I will display these antigens. This, along with signals from Th1, activate TC cells to release destructive perforins and granzymes. TC may also express which binds Fas on target cells inducing apoptosis.
Humoral Immunity

Figure 22.29: T-independent antigens: T-independent antigens have repeating epitopes that can induce B cell recognition and activation without involvement from T cells. A second signal, such as interaction of TLRs with PAMPs (not shown), is also required for activation of the B cell. Once activated, the B cell proliferates and differentiates into antibody-secreting s.

Figure 22.30: B Cell Activation: In T cell-dependent activation of B cells, the B cell recognizes and internalizes an antigen and presents it to a helper T cell that is specific to the same antigen. The helper T cell interacts with the antigen presented by the B cell, which activates the T cell and stimulates the release of cytokines that then activate the B cell. Activation of the B cell triggers proliferation and differentiation into B cells and plasma cells.
The second type of adaptive immunity involves stimulating B cells promoting the secretion of antibodies. When B cells bind antigen and receive signals from Th cells they become activated which includes proliferating and differentiating into plasma cells (antibody secreting B cells). Since antibodies are secreted out of the cell into the surrounding body fluid, this type of immunity is called (humors = bodily fluids). s are molecules that can stimulate B cells to differentiate into plasma cells without signals from Th cells. These are typically very large molecules with multiple, repeating epitopes (the specific region to which a receptor binds). T cell-independent antigens stimulate B cells by binding multiple BCRs simultaneously or through interactions with other surface proteins (such as the case with bacterial LPS). However, typically antigens are s.
Antibodies

Figure 22.31: B-cell receptors are embedded in the membranes of B cells. The variable regions of all of the receptors on a single cell bind the same specific antigen.
Antibodies are complex molecules made up of four protein subunits (two heavy chains and two light chains). The Y-shaped protein arms are flexible and contain the antigen-binding regions on the distal ends known as the (fragment of antigen binding, aka the ). This amino acid sequence varies greatly in this area such that there is a high likelihood for some antibodies to bind foreign antigens. The base of the protein, called the , contains the functional domain. This part helps activate complement and phagocytosis. Fc stands for “fragment of crystallization” since this protein fragment crystallizes after protein digestion. It may help to associate the “c” in Fc with the word “constant”, however, since this portion is referred to as the ..

Table 22.6: Table of Antibody Isotypes: IgD is bound to the membrane as the B cell receptor. All other isotypes are secreted forms.

Figure 22.32: Secondary antibody response: Compared to the primary response, the secondary antibody response occurs more quickly and produces antibody levels that are higher and more sustained. The secondary response mostly involves .
There are 5 distinct classes of Fc regions known as antibody isotypes. A B cell receptor is simply a membrane-bound antibody on the surface of the B cell known as . When a B cell becomes a plasma cell it secretes first. Before plasma cells have a chance to proliferate and greatly improve antigen binding, primary antibody-antigen interactions may be weak. To make up for this poor recognition, IgM has extra antigen binding sites because it is a pentamer (5 fused antibodies). As B cells proliferate they can undergo to more specialized isotypes and a stronger secondary response. IgG is the most common antibody found in plasma and can cross the placenta to provide an unborn baby with some adaptive immunity. is a dimer (2 fused antibodies) that is designed to cross plasma membranes. It is found in breast milk and the digestive tract. Finally, is an antibody often implicated in allergies due to its tendency to bind s on basophils and the similar mast cells.

Figure 22.33: Antibodies, especially IgM antibodies, agglutinate bacteria by binding to epitopes on two or more bacteria simultaneously. When multiple pathogens and antibodies are present, aggregates form when the binding sites of antibodies bind with separate pathogens.

Figure 22.34: Antibodies serve as opsonins and inhibit infection by tagging pathogens for destruction by macrophages, dendritic cells, and neutrophils. These phagocytic cells use Fc receptors to bind to IgG-opsonized pathogens and initiate the first step of attachment before phagocytosis.

Figure 22.35: Neutralization involves the binding of specific antibodies to antigens found on bacteria, viruses, and toxins, preventing them from attaching to target cells.
Antibodies facilitate immune functions by several methods. The flexible antigen-binding arms allow antibodies to induce (clumping) of pathogens, making them bigger targets, reducing mobility and the ability to adhere to body surfaces. Phagocytes such as neutrophils and macrophages can bind antibodies with Fc receptors enhancing phagocytosis. Thus, like C3b, antibodies facilitate (enhancing phagocytosis via opsonin coating). Finally, antibodies are relatively large proteins that can block biologically important molecules on the pathogen’s surface in a process of neutralization.
Development and Proliferation

Figure 22.36: Generating Lymphocyte Receptor Variability. (a) As a germ-line B cell matures, an enzyme called DNA recombinase randomly excises V and J segments from the light chain gene. Splicing at the mRNA level results in further gene rearrangement. As a result, (b) each antibody has a unique variable region capable of binding a different antigen.
The human genome only codes for approximately 20,000 protein genes. So, how does our immune system make the billions of different T and B cell receptors needed to bind any potential antigen? During development, different families of genes are ʻāwili (shuffled) and nucleotides randomly inserted to create a huge variety of lymphocyte receptors each with unique antigen-binding sites. This process is dependent on the action of . Remember all WBCs are born in the bone marrow. From there, T cells migrate to the thymus to mature whereas B cells remain in the bone marrow.

Figure 22.37: Differentiation of T Cells within the Thymus: Immature T-cells, called thymocytes, enter the thymus and go through a series of developmental stages that ensures both function and tolerance before they leave and become functional components of the adaptive immune response.
Both T and B cells undergo a similar two-step maturation process. The first step is called . During this phase, lymphocytes with the cell surface receptors that bind your MHC molecules receive signals to keep maturing. The second step is . Here, T or B cell receptors that bind your own antigens presented with MHC, called s, receive a negative signal and are destroyed. This is also known as because your immune system is customized to tolerate your self-antigens. Negative selection must be completed to prevent the formation of (pathologic adaptive immune responses against your own tissues).
Immunological Memory and Hypersomatic Mutation

Figure 22.38: Clonal Selection of B Cells: During a primary B cell immune response, both antibody-secreting plasma cells and memory B cells are produced. These memory cells lead to the differentiation of more plasma cells and memory B cells during secondary responses.
Activation and proliferation of T cells and B cells typically occurs in the cortical area of lymph nodes. s circulate the body through the cardiovascular system, entering lymph nodes along with lymph or more directly through . During , newly generated T cells have identical receptors as their parent cell. B cells proliferate as s within lymph node nodules, undergoing a process called . This allows genes encoding the antigen-binding regions of the B cell receptor (and antibody) to be altered. The selected B cells undergo clonal expansion with varying ability to bind the original antigen. The new clones that bind the antigen more strongly will again proliferate with hypersomatic mutation, and so on. In this way, the binding strength of the B cell receptor and secreted antibodies enhances over time. During an immune response, proliferating Th, Tc or B cells generate some . Potentially living decades, these cells can be readily reactivated providing long-term immunity (immunological memory).
![]() Retrieval Practice
Retrieval Practice
Survey of Immune System Cells
Imagine your skin is punctured by a thorn. This thorn isn’t sterile, it has bacteria on it. Describe your body’s immune responses to the damaged tissues and in the presence of a bacterial pathogen. Include events of inflammation, WBC recruitment, APC, and lymphocyte activities. Before looking back at the book or your notes, list all types of WBCs. Now look through the chapter and complete your list if you missed any. Now, wIthout looking at the book, list the function(s) of each WBC. Include details such as the reliance on PRRs or MHC molecules. This activity is comprehensive so you may want to break your work into categories such as innate and adaptive immune responses. Use the textbook to check all your work and make corrections.
Active vs. Passive and Natural vs Artificial Immunity

Figure 22.39: Primary and secondary immune adaptive response: This graph illustrates the primary and secondary immune responses related to antibody production after an initial and secondary exposure to an antigen. Notice that the secondary response is faster and provides a much higher concentration of antibody.
The first time you are exposed to an antigen, long-lived memory lymphocytes are generated providing an army of defense in case you are exposed to the same or similar pathogen again. This is your immune system’s . Your occurs upon reexposure. This time the immune system already has lots of pathogen-specific memory cells to prevent disease. However, there is a lag time between first exposure and a strong immune response. It takes time for Th cells to multiply and activate Tc and B cells. Then, these effector cells also have to multiply and produce memory cells. Upon a second exposure, even more memory cells and antibodies will be generated. Also, plasma cells will switch from producing IgM to the more effective IgG antibodies. Memory cells can impart immunological resistance to disease over your life span.

Figure 22.40: The four classifications of immunity. (credit top left photo: modification of work by USDA; credit top right photo: modification of work by “Michaelberry”/Wikimedia; credit bottom left photo: modification of work by Centers for Disease Control and Prevention; credit bottom right photo: Airman 1st Class Destinee Doughert / U.S. Air Force; Public Domain)
The generation of memory cells and antibodies due to exposure to an antigen is called . Active immunity can be natural or artificial. Active occurs when normal exposure to a pathogen or toxin causes the immune response. , such as vaccines, occurs when an antigen is intentionally injected into the body for the sake of generating antibodies and memory lymphocytes. In either case, it usually takes a few weeks for active immunity to reach full strength. occurs when antibodies are transferred from one individual to another. This occurs naturally during pregnancy and breastfeeding. During pregnancy, IgG antibodies can cross from the maternal to fetal tissues of the placenta. IgA antibodies are found in breastmilk. The transfer of these antibodies imparts the adaptive immunity profile of the mother to the child whose immune system hasn’t yet developed a strong repertoire of mature lymphocytes. There is also passive artificial immunity, which occurs when antibodies are transferred from one person to another. This has traditionally been done using serum from someone previously exposed to a pathogen to prevent disease in another.
22.5 Specific Diseases
![]() 22.5 Learning Outcomes
22.5 Learning Outcomes
- Define antigenic, immunogenic, infectious, endemic, epidemic, pandemic, morbidity and mortality
- Relate specific diseases to specific pathogen or homeostatic imbalance
- Compare and contrast disease etiology, symptoms, prophylaxis, and treatment
As we have learned, foreign antigen bound to MHC is required for an effective adaptive immunity response. Not all foreign molecules have equal (capable of binding T or B cell receptors). Once more, a foreign molecule with high antigenicity may not have a high degree of (capable of initiating effective immune responses). If a pathogen is able to avoid destruction by your immune system, it may cause disease. Not all diseases are (able to spread from person to person). Some diseases are (common within a population). Usually this means that the population as a whole will have some resistance to the pathogen in the form of antibodies and memory lymphocytes despite it persisting at a relatively constant level. If an infectious disease spreads to a new area where a population has no immunity, it may spread very quickly causing an maʻi ahulau (). In some cases, newly emerging pathogens will spread throughout the world population causing a (pan = all).
Rat Lungworm Disease
 |
 |
| The life cycle of angiostrongyliasis | Adult female worm of Angiostrongylus cantonensis recovered from rat lungs with characteristic barber-pole appearance (anterior end of worm is to the top). Scale bar = 1 mm. |
Figure 22.41: Angiostrongylus cantonensi
Rat lungworm disease, or , is a disease that affects the central nervous system. Humans get infected after ingestion or exposure to the larvae of a parasitic nematode (roundworm parasite), Angiostrongylus cantonensis. The larvae are eaten by slugs or snails. In Hawaii, they are commonly found on raw, uncooked leafy green vegetables. The presence of the parasite in the human body results in a rare type of meningitis, that ranges from mild to severe. Research is still ongoing, but the infected meninges is linked to increased eosinophil count, which helps to kill the parasites but may result in an increased acute inflammatory response. Symptoms include severe headache and stiffness of the neck, tingling or painful feelings in the skin or extremities, low-grade fever, nausea, and vomiting starting 1-3 weeks after infection and can last for months or even years. It appears that individuals can get infected with rat lungworm disease more than once, indicating limited immunity to the pathogen that causes it.
Acquired Immune Deficiency Syndrome (AIDS)
 |
 |
| A diagram including the early symptoms of HIV ( Human Immunodeficiency Virus) and where it appears in the body. | The HIV replication cycle |
Figure 22.42: HIV Symptoms and Replication
The is an infection that may lead to , a serious and lethal disease characterized by a greatly weakened immune system. The virus is transmitted through semen, vaginal fluids, and blood. In the United States, HIV is mainly transmitted by having sex or sharing syringes and needles with someone who is infected. Like all viruses, HIV needs living host cells to replicate. Upon introduction to the body, it sticks to target cells and tricks the target cells into internalizing it. Once in the host cell, this virus utilizes reverse transcriptase, using its viral RNA to synthesize DNA. This process is opposite to the normal process of genetic transcription and thus viruses that replicate in this manner are called retroviruses.

Figure 22.43: HIV Disease Progression Seroconversion, the rise of anti-HIV antibody levels and the concomitant decline in measurable virus levels, happens during the first several months of HIV disease. Unfortunately, this antibody response is ineffective at controlling the disease, as seen by the progression of the disease towards AIDS, in which all adaptive immune responses are compromised.
Sometimes, but not always, flu-like symptoms occur in the first 1 to 2 weeks after infection. This is later followed by . Seroconversion is the reciprocal relationship between virus levels in the blood and antibody levels. As the antibody levels rise, the virus levels decline, and this is a sign that the immune response is at least partially effective. The anti-HIV antibodies formed during seroconversion are the basis for most initial HIV screening done in the United States. Because the seroconversion time varies between individuals, a second test is given months after the first to confirm a negative result.
After seroconversion, the amount of virus circulating in the blood drops and stays low for several years. During this time, the levels of CD4 T helper cells decline steadily until the immune response is so weak that begin leading to death. HIV needs the CD4 and other receptors to get inside cells. HIV, therefore, severely hinders CD4 T helper cells which are required for both branches of the adaptive immune system; cell-mediated and humoral immunity.
Treatment for the disease consists of drugs that target virally encoded proteins that are necessary for viral replication but are absent from normal human cells. This approach has been successful in significantly prolonging the lives of HIV-positive individuals. Unfortunately, HIV is good at escaping recognition by the immune system. It mutates surface antigens at a high rate making it difficult to mount a strong immune response. HIV vaccines have been in development for 30 years and still none have proven adequately effective.
Coronavirus

Figure 22.44: Coronavirus: Colorized scanning electron micrograph of a cell (teal and green) infected with a variant strain of SARS-CoV-2 virus particles (UK B.1.1.7- purple and pink), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
Coronaviruses are a family of RNA viruses found in various types of animals. They are called coronaviruses due to their resemblance to the lā (sun). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel strain responsible for coronavirus disease-2019 () that was officially declared a worldwide pandemic in the year 2020. The fact that this infectious virus can be transmitted via respiratory droplets and possibly even aerosols by both symptomatic and asymptomatic people has made it difficult to control. COVID-19 affects individuals differently and symptoms range from mild to severe. Symptoms may include a cough, head and body aches, and sometimes loss of taste and/or smell. Most of those infected recover in about 10 days just as adaptive immune responses are reaching full strength. In more severe cases, pneumonia, respiratory failure, shock and death may occur. Age correlates strongly with disease severity with the elderly having the worst . By January 2023, the CDC reported more than 100 million Americans had acquired COVID-19 with just over one million deaths.
Allergies and hypersensitivities

Figure 22.45: Immunological hypersensitivity: On first exposure to an allergen in a susceptible individual, antigen-presenting cells process and present allergen epitopes with major histocompatibility complex (MHC) II to T helper cells. B cells also process and present the same allergen epitope to TH2 cells, which release cytokines IL-4 and IL-13 to stimulate proliferation and differentiation into IgE-secreting plasma cells. IgE binds to mast cell Fc receptors. Upon subsequent exposure, the allergen cross-links mast cell-bound IgE causing the release of granules (degranulation) resulting in the symptoms of type I reactions.
Immunological hypersensitivity is an excessive, potentially harmful overreaction to an antigen. There are four types. () involves antigens called . Upon first exposure to an allergen a susceptible person will often be asymptomatic but develop anti-allergen IgE antibodies that bind receptors on mast cells and basophils. Subsequent exposure to the same allergen causes mast cells to release histamine and other chemicals, increasing inflammation resulting in the symptoms commonly associated with an allergic reaction.
Symptoms of inhaled allergens from substances such as mold, dust, pollen, and animal dander are nasal edema and runny nose caused by the increased vascular permeability and increased blood flow of nasal blood vessels. Most allergens are in themselves nonpathogenic and therefore mostly harmless. Some individuals develop mild allergies, which are usually treated with s. However, others develop severe allergies in response to allergens in bee and wasp venoms, toxins in plants, and some foods such as nuts, milk, eggs, and shellfish. Severe allergies may cause anaphylactic shock, which can potentially be fatal within 20 to 30 minutes if untreated. Because epinephrine raises blood pressure and relaxes bronchial smooth muscle, it is routinely used to counteract the effects of anaphylaxis and can be lifesaving. Patients with known severe allergies are encouraged to always keep automatic epinephrine injectors with them, especially when away from easy access to professional emergency healthcare.

Figure 22.46: Skin-Prick Test: Results of an allergy skin-prick test to test for type I hypersensitivity to a group of potential allergens. A positive result is indicated by a raised area (wheal) and surrounding redness (flare). (credit: modification of work by “OakleyOriginals”/Flickr)

Figure 22.47: Type III hypersensitivities and the systems they affect. (a) Immune complexes form and deposit in tissue. Complement activation, stimulation of an inflammatory response, and recruitment and activation of neutrophils result in damage to blood vessels, heart tissue, joints, skin, and/or kidneys. (b) If the kidneys are damaged by a reaction, dialysis may be required. (Credit b:Tech. Sgt. Bennie J. Davis III / US Air Force; Public Domain)
Allergists use skin testing to identify allergens in some types of hypersensitivities. In skin testing, allergen extracts are injected into the epidermis. A positive result typically manifests within 30 minutes as a soft, pale swelling at the site surrounded by a red zone called the wheal and flare response caused by the release of histamine and the granule mediators. The soft center is due to fluid leaking from the blood vessels and the redness is caused by the increased blood flow that results from the dilation of local blood vessels.

Figure 22.48: Type IV Hypersensitivity: Exposure to hapten antigens in poison ivy can cause contact dermatitis, a type IV hypersensitivity. (a) The first exposure to poison ivy does not result in a reaction. However, sensitization stimulates helper T cells, leading to production of memory helper T cells that can become reactivated on future exposures. (b) Upon secondary exposure, the memory helper T cells become reactivated, producing inflammatory cytokines that stimulate macrophages and cytotoxic T cells to induce an inflammatory lesion at the exposed site. This lesion, which will persist until the allergen is removed, can inflict significant tissue damage if it continues long enough.
involves IgG and IgM antibodies that bind your own cells leading to complement activation and subsequent cellular destruction via the MAC. Type III hypersensitivity also involves IgG antibodies but here immune complexes are formed that cause localized inflammation often in the joints or kidney. Finally, (aka ) is an antibody-independent response with tissue damage being primarily caused by T cells and macrophages.

Figure 22.49: Types of Hypersensitivities: Components of the immune system cause four types of hypersensitivities. Notice that types I–III are B-cell/antibodymediated hypersensitivities, whereas type IV hypersensitivity is exclusively a T-cell phenomenon.
Autoimmune

Figure 22.50: Goiter, a hypertrophy of the thyroid, is a symptom of Graves disease and Hashimoto thyroiditis.

Figure 22.51: Exophthalmia, or Graves ophthalmopathy, is a sign of Graves disease. (credit: modification of work by Jonathan Trobe, University of Michigan Kellogg Eye Center)

Figure 22.52: Rheumatoid arthritis: The radiograph (left) and photograph (right) show damage to the hands typical of rheumatoid arthritis. (credit right: modification of work by “handarmdoc”/Flickr)

Figure 22.53: Lupus: SLE. (a) Systemic lupus erythematosus is characterized by autoimmunity to the individual’s own DNA and/or proteins. (b) This patient is presenting with a butterfly rash, one of the characteristic signs of lupus.
Sometimes, the adaptive immune system will erroneously attack your body’s healthy cells. When this happens, the person is said to have an autoimmune disease. The trigger for these diseases is often unknown with both environmental and genetic factors suspected in most cases. Treatments are usually based on resolving the symptoms using immunosuppressive and anti-inflammatory drugs such as steroids. The symptoms vary depending on the specific type of autoimmune disease. For instance, is an autoimmune disease that causes pain, stiffness, and loss of function of the joints. It is associated with high levels of IL-1, IL-6, TNF-𝜶 which may cause inflammation of the synovial membranes among other tissues. , an autoimmune disease often involves anti-nuclear protein antibodies resulting in systemic inflammation. Symptoms include joint aches, fever, fatigue in addition to the characteristic skin rashes and swelling. is the most common cause of hyperthyroidism. It is caused by thyroid stimulating antibodies mimicking TSH. Irritability, sleeping problems, tachycardia, heat sensitivity, weight loss, eye bulging and other eye problems are common. Treatments may include removal of the thyroid followed by life-long hormone treatment.
Environmental triggers seem to play a large role in autoimmune responses. One explanation for the breakdown of central tolerance is that, after certain bacterial infections, an immune response to a component of the bacterium cross-reacts with a self-antigen. This mechanism is seen in as a result of infection with Streptococcus, the etiologic agent of strep throat. The antibodies generated to this pathogen’s M protein cross-react with an antigenic component of heart myosin, a major contractile protein of the heart that is critical to its normal function. The antibody binds to these molecules and activates complement proteins, causing inflammation of the heart, especially to the heart valves.
Despite the link between bacteria and rheumatic fever, some theories propose that exposure to a diversity of common pathogens may reduce the prevalence of hypersensitivities and autoimmune responses. The fact that allergies and autoimmune diseases are rare in countries that have a high incidence of infectious diseases supports this idea. The overuse of antimicrobials in soaps, toys and other products can compromise the healthy microbiota, promote antibiotic resistance, in addition to preventing your immune system from being properly primed to the antigens in the surrounding environment.
| Select Autoimmune Diseases | ||
|---|---|---|
| Disease | Cause | Signs and Symptoms |
| Addison disease | Destruction of adrenal gland cells by cytotoxic T cells | Weakness, nausea, hypotension, fatigue; adrenal crisis with severe pain in abdomen, lower back, and legs; circulatory system collapse, kidney failure |
| Celiac disease | Antibodies to gluten become autoantibodies that target cells of the small intestine | Severe diarrhea, abdominal pain, anemia, malnutrition |
| Diabetes mellitus (type I) | Cytotoxic T-cell destruction of the insulin-producing β cells of the pancreas | Hyperglycemia, extreme increase in thirst and urination, weight loss, extreme fatigue |
| Graves disease | Autoantibodies target thyroid-stimulating hormone receptors, resulting in overstimulation of the thyroid | Hyperthyroidism with rapid and irregular heartbeat, heat intolerance, weight loss, goiter, exophthalmia |
| Hashimoto thyroiditis | Thyroid gland is attacked by cytotoxic T cells, lymphocytes, macrophages, and autoantibodies | Thyroiditis with goiter, cold intolerance, muscle weakness, painful and stiff joints, depression, memory loss |
| Multiple sclerosis (MS) | Cytotoxic T-cell destruction of the myelin sheath surrounding nerve axons in the central nervous system | Visual disturbances, muscle weakness, impaired coordination and balance, numbness, prickling or “pins and needles” sensations, impaired cognitive function and memory |
| Myasthenia gravis | Autoantibodies directed against acetylcholine receptors within the neuromuscular junction | Extreme muscle weakness eventually leading to fatal respiratory arrest |
| Psoriasis | Cytokine activation of keratinocytes causes rapid and excessive epidermal cell turnover | Itchy or sore patches of thick, red skin with silvery scales; commonly affects elbows, knees, scalp, back, face, palms, feet |
| Rheumatoid arthritis | Autoantibodies, immune complexes, complement activation, phagocytes, and T cells damage membranes and bone in joints | Joint inflammation, pain and disfigurement, chronic systemic inflammation |
| Systemic lupus erythematosus (SLE) | Autoantibodies directed against nuclear and cytoplasmic molecules form immune complexes that deposit in tissues. Phagocytic cells and complement activation cause tissue damage and inflammation | Fatigue, fever, joint pain and swelling, hair loss, anemia, clotting, a sunlight-sensitive “butterfly” rash, skin lesions, photosensitivity, decreased kidney function, memory loss, confusion, depression |
Table 22.7: Autoimmune Diseases – OpenStax Microbiology
Stress and the Immune System
The human response to stress includes both physical and psychological symptoms. Acute (short term) stress can be beneficial such as that brought on by exercise. Chronic stress (an ongoing state of physiological arousal), however, has been linked to increased illness. Chronic stress activates the sympathetic (“fight or flight”) division of the autonomic nervous system. Through a cascade of physiological events, sympathetic stimulation results in the release of cortisol (the “stress” hormone). Persistent high levels of cortisol reduces levels of immature B cells. Decreased levels of B cells result in reduced antibody production in the body, impairing the adaptive immune response. Additionally, norepinephrine release associated with activation of the sympathetic nervous system further facilitates inflammation by promoting the release of inflammatory mediators. This combination of dampened adaptive immune function and constant inflammation makes the body less efficient at controlling pathogens while also causing damage to self tissues. Being more susceptible to disease and the discomfort of inflammation exacerbates the stress level, thus further suppressing adaptive immunity and increasing inflammation in a negative spiral.
It has been reported that chronic inflammation is linked to more than 50% of all deaths worldwide. Poor diet, obesity, physical inactivity, chronic stress, environmental pollutants, and poor sleep are common causes of systemic chronic inflammation that can lead to cardiovascular disease, cancer, diabetes mellitus, kidney disease, and autoimmune and neurodegenerative disorders. Prevention of chronic inflammation includes conscious lifestyle changes (increasing exercise and eating a plant-based whole foods diet) and a focus on positive changes to both the social and physical environment.
is the field of study that focuses on the link between the immune, nervous and endocrine systems. Physical disorders or diseases whose symptoms are brought about or worsened by stress and emotional factors are called . A list of frequently encountered psychophysiological disorders is provided in the following table:
| Type of Psychophysiological Disorder | Examples |
|---|---|
| Cardiovascular | hypertension, coronary heart disease |
| Gastrointestinal | irritable bowel syndrome |
| Respiratory | asthma, allergy |
| Musculoskeletal | low back pain, tension headaches |
| Skin | acne, eczema, psoriasis |
Table 22.8: Types of Psychophysiological Disorders (adapted from Everly & Lating, 2002) – OpenStax Psychology 2e
The mechanism whereby stress causes inflammation is thought to involve the stress hormone cortisol promoting cytokine production. This results in chronic innate immune system responses. Another potential link involves . CRP, a protein synthesized by the liver, is secreted into the bloodstream in response to inflammation. Elevated CRP is found in individuals with cardiovascular disease (CVD) and recent studies also revealed a correlation between CRP and work-related stress. Reducing stress as a possible means of reducing chronic inflammation and associated psychophysiological disorders may be an important step in improving immune system function.

Chapter Summary
Quiz
Citations:
ʻŌlelo Noʻeau — Pukui #1252
https://doi.org/10.1006/jhge.2001.0325 and cdc.gov/leprosy
https://doi.org/10.3389/fimmu.2021.646333
Liu YZ, Wang YX, Jiang CL. Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci. 2017;11:316. Published 2017 Jun 20. doi:10.3389/fnhum.2017.00316
https://www.cdc.gov/hiv/risk/index.html
McGregor BA, Murphy KM, Albano DL, Ceballos RM. Stress, cortisol, and B lymphocytes: a novel approach to understanding academic stress and immune function. Stress. 2016;19(2):185-191. doi:10.3109/10253890.2015.1127913
Citations:
Bancos, S., Bernard, M. P., Topham, D. J., & Phipps, R. P. (2009). Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cellular immunology, 258(1), 18–28. https://doi.org/10.1016/j.cellimm.2009.03.007
Minta, J. O., Urowitz, M. B., Smythe, H. A., & Isenman, D. E. (1983). Effect on the human complement system of the major non-steroidal anti-inflammatory drugs: aspirin, indomethacin, phenylbutazone, oxyphenbutazone, and sulindac. Clinical and experimental immunology, 53(3), 555–561.
Citation: Prasad A. S. (2008). Zinc in human health: effect of zinc on immune cells. Molecular medicine (Cambridge, Mass.), 14(5-6), 353–357. https://doi.org/10.2119/2008-00033.Prasad
Citation: Furman, D., Campisi, J., Verdin, E. et al. Chronic inflammation in the etiology of disease across the lifespan. Nat Med 25, 1822–1832 (2019). https://doi.org/10.1038/s41591-019-0675-0

Key Terms
acquired immunodeficiency syndrome (AIDS)
a serious and lethal disease caused by human immunodeficiency virus (HIV) and characterized by a greatly weakened immune system
active immunity
immunity developed from an individual’s own immune system
adaptive immunity
relatively slow but very specific and effective immune response involving lymphocytes
afferent lymphatic vessels
lead into a lymph node
agglutination
clumping, the amassing of cells due to antibody binding
agranulocytes
WBCs that aren’t granulocytes including monocytes, dendritic cells and lymphocytes
allergens
antigens responsible for type I hypersensitivity
anchoring filaments
projecting, hair-like structures that prevent lymphatic capillary collapse
angiostrongyliasis
rat lungworm disease, caused by the parasitic nematode Angiostrongylus cantonensis carried by slugs and snails
antibody
antigen-specific protein secreted by plasma cells; immunoglobulin
antigen
molecule recognized by the receptors of B and T lymphocytes
antigenicity
the degree to which an antigen binds a T or B cell receptor
antigen presentation
binding of processed antigen to the protein-binding cleft of a major histocompatibility complex molecule
antihistamine
a drug that counteracts the effects of histamine
antimicrobial peptides
a relatively short chain of amino acids with anti-bacterial, -viral or -fungal properties
antipyretic
fever-reducing medications
APC
an antigen presenting cells, namely macrophages and dendritic cells
artificial immunity
occurs when an antigen is intentionally injected into the body for the sake of generating antibodies and memory lymphocytes such as with vaccines
autoimmune diseases
a pathologic adaptive immune response against your own tissues
bacteria
single-celled, prokaryotic microorganisms
basophil
granulocytes with histidine containing granules and are implicated in allergies
bronchus-associated lymphoid tissue (BALT)
bronchus-associated lymphoid tissue, lymphoid nodule associated with the respiratory tract
B cell
lymphocyte that acts by differentiating into an antibody-secreting plasma cell
C3
complement protein that fragments into C3a and C3b. C3a enhances inflammation and C3b is an opsonin and activates C5
C5
complement protein that fragments into C5a and C5b. C5a enhances inflammation C5b initiates formation of the MAC by triggering the interaction of C6, C7, C8 and several C9s
CAMs
cell adhesion molecules that promote intercellular binding
CD4+ T cells
Th , helper T cells, T cells that express the surface protein CD4
central tolerance
B cell tolerance induced in immature B cells of the bone marrow
cell-mediated immunity
adaptive immunity activated by helper T cell subclass Th1, involving TC
chemokine
soluble, long-range, cell-to-cell communication molecule
chemotaxis
cells following a chemical concentration gradient such as leukocytes following a chemokine trail toward a site of injury
chyle
lipid-rich lymph inside the lymphatic capillaries of the small intestine
cisterna chyli
bag-like vessel that forms the beginning of the thoracic duct
classical pathway
complement proteins are activated by the presence of pathogen bound antibodies
class switching
ability of B cells to change the class of antibody they produce without altering the
specificity for antigen, aka isotype switching
clonal expansion
proliferation of B lymphocytes with a specific antigen receptor into a population with varying degree of antigen binding strength
complement
enzymatic cascade of constitutive blood proteins that have anti-pathogen effects, including
the direct killing of bacteria
constant region
domain part of a lymphocyte antigen receptor that does not vary much between different
receptor types
COVID-19
coronavirus disease-2019 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for a worldwide pandemic in the year 2020
C-Reactive Protein (CRP)
a protein associated with stress, inflammation and psychophysiological disorders
CSFs
colony-stimulating factors, induce proliferation of WBCs in the bone marrow
cytokine
soluble, short-range, cell-to-cell communication molecule
cytotoxic T cells (Tc)
T lymphocytes with the ability to induce apoptosis in target cells
DAMPs
damage-associated molecular patterns recognized by PRRs of innate immunity
defensin
a chemical class type of AMPs
delayed hypersensitivity (type IV)
T cell-mediated immune response against pathogens infiltrating interstitial tissues, causing cellular infiltrate
dendritic cell
an APC similar to macrophages
dermcidin
a chemical class type of AMPs
diapedesis
leukocytes squeezing between endothelial, out of the blood into the surrounding tissues
disease
abnormal functioning part of the body, illness, sickness or ailment
effector B cells
plasma cells, activated B lymphocytes
effector T cells
immune cells with a direct, adverse effect on a pathogen
efferent lymphatic vessels
lead out of a lymph node
endemic
a disease common within a population
eosinophil
granulocytes specialised for attaching parasites such as worms
epidemic
the rapid spreading or temporary prevalence of disease in a population
epitope
the specific region of an antigen that binds to a T or B cell receptor
Fab
fragment of antigen binding, that part of the antibody that binds antigen
fas ligand (FasL)
molecule expressed on cytotoxic T cells and NK cells that binds to the fas molecule on a
target cell and induces it do undergo apoptosis
Fc receptor
a membrane bound protein on phagocytes that binds the Fc region of an antibody
Fc region
in an antibody molecule, the site where the two termini of the heavy chains come together;
many cells have receptors for this portion of the antibody, adding functionality to these molecules
fever
abnormally high body temperature, aka pyrexia
fungi
eukaryotic organisms distinguished from animals and plants by chitin-containing cell walls among other features
germinal center
clusters of rapidly proliferating B cells found in secondary lymphoid tissues
granzyme
apoptosis-inducing substance contained in granules of NK cells and cytotoxic T cells
granulocytes
WBCs that have granules; neutrophils, eosinophils and basophils
Grave’s disease
an autoimmune disease causing hyperthyroidism resulting in tachycardia, heat sensitivity, weight loss, eye bulging among other symptoms
Hansen’s disease (leprosy)
a disease caused by a Mycobacterium leprae infection primarily affecting the peripheral nervous system, skin, and nasal mucosa
helper T cells (Th)
T cells that secrete cytokines to enhance other immune responses, involved in
activation of both B and Tc cell lymphocytes
high endothelial venules (HEV)
vessels containing unique endothelial cells specialized to allow migration of
lymphocytes from the blood to the lymph node
histamine
vasoactive mediator in granules of basophils and mast cells and is the primary cause of allergies and anaphylactic shock
human immunodeficiency virus (HIV)
is a virus capable of causing acquired immunodeficiency syndrome (AIDS), a serious and lethal disease characterized by a greatly weakened immune system
humoral immunity
adaptive immunity involving Th2 stimulating B cells to secrete antibodies
hypersomatic mutation
the process of the antigen-binding regions of the B cell receptor (and antibody) to be altered during B cell clonal expansion
hyperemia
increased blood flow near the site of inflammation
hypersensitivity
an excessive, potentially harmful overreaction to an antigen
IgA
antibody whose dimer is secreted by exocrine glands, is especially effective against digestive and respiratory pathogens, and can pass immunity to an infant through breastfeeding
IgD
class of antibody whose only known function is as a receptor on naive B cells; important in B cell activation
IgE
antibody that binds to mast cells and causes antigen-specific degranulation during an allergic response
IgG
main blood antibody of late primary and early secondary responses; passed from carrier to unborn child via placenta
IgM
antibody whose monomer is a surface receptor of naive B cells; the pentamer is the first antibody made blood plasma during primary responses
IL-4
interleukin-4, promotes Th2 and plasma cell development among other functions
immediate hypersensitivity (type I)
IgE-mediated mast cell degranulation caused by crosslinking of surface IgE by antigen
immune system series of barriers, cells, and soluble mediators that combine to response to infections of the body with pathogenic organisms
immunogenicity
the degree by which an antigen induces an immune response
immunoglobulin
protein antibody; occurs as one of five main classes
immunological memory
ability of the adaptive immune response to mount a stronger and faster immune response upon re-exposure to a pathogen
infectious
a disease capable of transmitting from person to person
inflammation
basic innate immune response characterized by heat, redness, pain, and swelling
innate immunity
rapid but relatively nonspecific immune responses
interferons (INFs)
early induced proteins made in virally infected cells that cause nearby cells to make antiviral proteins
interleukins
chemical messengers released by WBCs to stimulate others in an autocrine or paracrine manner
interstitial fluid (IF)
fluid between cells and tissues, excluding plasma or lymph
kinins
peptides that contribute to pain, inflammation and smooth muscle contraction
lacteal
lymphatic capillaries of the gut that absorb bile to form chyle
lectin pathway
complement activated by the presence of certain carbohydrates typical of some bacteria
leukocyte (aka WBC)
white blood cells, cells of the immune system
leukocytosis
a high WBC count
leukopoiesis
leukocyte proliferation in bone marrow
lymph
fluid contained within the lymphatic system
lymph node
one of the bean-shaped organs found associated with the lymphatic vessels
lymphatic capillaries
smallest of the lymphatic vessels and the origin of lymph flow
lymphatic system
network of lymphatic vessels, lymph nodes, and ducts that carries lymph from the tissues and back to the bloodstream.
lymphatic trunks
large lymphatics that collect lymph from smaller lymphatic vessels and empties into the
blood via lymphatic ducts
lymphatic vessels
Lymphatic vasculature that form from fused smaller lymphatic capillaries, these deliver lymph to and from lymph nodes
lymphocytes
white blood cells characterized by a large nucleus and small rim of cytoplasm
MAC
membrane attack complex, the interaction of C5, C6, C7, C8 and several C9s creates a hole in bacterial membranes disrupting the targeted cell
macrophage
ameboid phagocyte found in several tissues throughout the body
major histocompatibility complex (MHC)
gene cluster whose proteins present antigens to T cells
MALT
mucosa-associated lymphoid tissue, lymphoid tissue associated with the digestive tract and respiratory system
margination
instead of the middle of the blood vessel, cells travel along the wall by sticking and rolling via CAM interactions
mast cell
cell found in the skin and the lining of body cells that contains cytoplasmic granules with
vasoactive mediators such as histamine (similar to basophils)
memory lymphocytes
long-lived T or B cell reserved for future exposure to a pathogen
MHC class I
found on most cells of the body, it binds to the CD8 molecule on T cells
MHC polygeny
multiple MHC genes and their proteins found in body cells
MHC polymorphism
multiple alleles for each individual MHC locus
microbiota (aka normal flora)
the typical bacteria, viruses and other microbes associated with healthy populations
mini flaps
openings that allow IF to enter lymphatic capillaries, becoming lymph
monocyte
precursor to macrophages and dendritic cells seen in the blood
naïve lymphocyte
mature B or T cell that has not yet encountered antigen for the first time
natural killer cell (NK)
cytotoxic lymphocyte of innate immune response
natural immunity
the normal exposure to a pathogen or toxin causing an immune response
negative selection
selection against thymocytes in the thymus that react with self-antigen neutralization inactivation of a virus by the binding of specific antibody
neutralization
antibodies blocking disease promoting molecules on a pathogens such as adherence proteins
neutrophil
phagocytic white blood cell recruited from the bloodstream to the site of infection via the
bloodstream
NETs
neutrophil extracellular traps, released DNA that traps and thereby sequestering pathogens
NSAIDs
non-steroidal anti-inflammatory drugs such as aspirin and ibuprofen
opportunistic diseases
pathogens that don’t normally cause disease in healthy individuals but can when the immune system is compromised in some way
opsonization
enhancement of phagocytosis by the binding of antibody or antimicrobial protein
PAMPS
pathogen associated molecular patterns that are recognized by PRRs of the innate immune system
pandemic
an epidemic of a large area such as an entire country, continent, or planet
paresthesia
an abnormal sensation, such as prickling, itching, etc.
passive immunity
transfer of immunity to a pathogen to an individual that lacks immunity to this pathogen
usually by the injection of antibodies
pathogen
a pathology-inducing agent such as certain bacteria, fungi or viruses
pathogenicity
the extent or ability of a pathogen to cause disease
pattern recognition receptor (PRR)
leukocyte receptor that binds to specific cell wall components of different bacterial species
perforin molecule in NK cell and cytotoxic T cell granules that form pores in the membrane of a target cell
perforin
an enzyme that forms holes in the plasma membrane, released by NK and Tc cells
Peyer’s patches
lymphoid follicles associated with distal regions of the small intestine, part of the MALT
phagocyte
a cell capable of phagocytosis, namely neutrophils, macrophages and dendritic cells
phagocytosis
movement of material from the outside to the inside of the cells via vesicles made from
invaginations of the plasma membrane
plasma cell
differentiated B cell that is actively secreting antibody
PMN
polymorphonuclear cells, aka granulocytes
positive selection
selection of thymocytes within the thymus that interact with self, but not non-self, MHC molecules
primary adaptive response
immune system’s response to the first exposure to a pathogen
primary lymphoid organ
site where lymphocytes mature and proliferate; red bone marrow and
thymus gland
prion
a protein folded into an abnormal shape that causes normal proteins to also misfold
prognosis
forecasting the probable outcome of disease including chances of recovery
prostaglandins
lipids associated with inflammation, bronchodilation and many other effects
PRRs (pattern recognition receptors)
receptors on cells of the innate immune system that bind PAMPs and DAMPs
psychoneuroimmunology
study of the connections between the immune, nervous, and endocrine
systems
psychophysiological disorders
diseases whose symptoms are brought about or worsened by stress and emotional factors
pyrexia
abnormally high body temperature, aka fever
pyrogens
chemicals that signal the hypothalamus to increase body temperature
recombination-activating genes (RAGs)
the genes responsible for recombining segments and randomly inserting nucleotides into the variable regions of T and B cell receptor genes
red pulp
areas within the spleen where old, damaged RBCs are broken down
regulatory T cells (Treg, suppressor T cells)
class of CD4 T cells that regulates other T cell responses
respiratory pump
the mechanism by which plasma and lymph is drawn toward the heart due to the low pressure in the thoracic cage created by the respiratory system
rheumatoid arthritis (RA) is an autoimmune disease that causes inflammation, pain, stiffness, and loss of function of the joints
rheumatic fever
an infection with Streptococcus resulting in inflammation heart, skin, joints or brain tissue
right lymphatic duct
drains lymph fluid from the upper right side of body into the right subclavian
vein
secondary adaptive response
immune response observed upon re-exposure to a pathogen, which is
stronger and faster than a primary response
secondary lymphoid organ
sites where lymphocytes mount adaptive immune responses; examples include lymph nodes and spleen
self-antigen
molecules produced by and that do not induce adaptive immune responses of an individual in the absence of autoimmune disease
seroconversion
clearance of pathogen in the serum and the simultaneous rise of serum antibody
systemic lupus erythematosus (SLE)
an autoimmune disease causing inflammation, joint aches, fever, and characteristic skin rashes and swelling
T cell
a cell type of the adaptive immune system, the two main subtypes being TH and Tc
T cell-dependent antigen
antigen that binds to B cells, which requires signals from T cells to make antibody
T cell-independent antigen
binds to B cells, which do not require signals from T cells to make antibody
Th1
cells that secrete cytokines that enhance the activity of Tc
Th2
cells that secrete cytokines that induce B cells to differentiate into plasma cells
thoracic duct
large duct that drains lymph from the lower limbs, left thorax, left upper limb, and the left
side of the head
thymus
primary lymphoid organ; where T lymphocytes proliferate and mature
tonsils
lymphoid nodules associated with the nasopharynx
type I hypersensitivity
immediate response mediated by mast cell degranulation caused by the crosslinking of the antigen-specific IgE molecules on the mast cell surface
type II hypersensitivity
cell damage caused by the binding of antibody and the activation of complement, usually against red blood cells
type III hypersensitivity
damage to tissues caused by the deposition of antibody-antigen (immune) complexes followed by the activation of complement
type IV hypersensitivity (aka delayed hypersensitivity)
an antibody-independent response primarily involving by T cells and macrophages
valves
inward projections within lymphatic capillaries that prevent lymph backflow
variable region
domain part of a lymphocyte antigen receptor that varies considerably between different
receptor types
vasodilation
an increase in blood vessel diameter
virus
a non-living infectious agent that requires a living cell to multiply
white pulp
areas in the spleen where numerous WBCs are performing immunological functions
Media Attributions
- olelo_noeau
- Kealakekua Bay © John Webber is licensed under a Public Domain license
- Relative Size © OpenStax is licensed under a CC BY (Attribution) license
- The Bishop Home for Boys, Kalauau, Molokai © Unknown - http://chroniclingamerica.loc.gov/lccn/sn88085187/1904-05-07/ed-1/seq-3/ is licensed under a Public Domain license
- Hansen’s Disease © OpenStax is licensed under a CC BY (Attribution) license
- Tinea Versicolor © Sarahrosenau on Flickr.com is licensed under a CC BY-SA (Attribution ShareAlike) license
- Lymphatic Capillaries © OpenStax is licensed under a CC BY (Attribution) license
- Lymph Capillaries and Pressure gradient: Precapillary Sphincters and Valves © OpenStax is licensed under a CC BY (Attribution) license
- Lymph Capillaries and Pressure gradient: Lymph Capillaries in the Tissue Spaces © OpenStax is licensed under a CC BY (Attribution) license
- Lymph Capillaries and Pressure Gradient: Capillary Exchange © OpenStax is licensed under a CC BY (Attribution) license
- Lymph Nodes, Lymphatic Trunks and Ducts: Location, Structure, and Histology of the Thymus © OpenStax is licensed under a CC BY (Attribution) license
- Lymph Nodes, Lymphatic Trunks and Ducts: Major Trunks and Ducts of the Lymphatic System © OpenStax is licensed under a CC BY (Attribution) license
- Lymph Nodes, Lymphatic Trunks and Ducts: Structure and Histology of a Lymph Node © OpenStax is licensed under a CC BY (Attribution) license
- Lymph Nodes, Lymphatic Trunks and Ducts: Mucosa-associated Lymphoid Tissue (MALT) Nodule © OpenStax is licensed under a CC BY (Attribution) license
- Lymphatic System Trunks © Henry Vandyke Carter in Grey's Anatomy is licensed under a Public Domain license
- Red Bone Marrow © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- The Thymus © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- The Spleen © OpenStax AP Bio is licensed under a CC BY (Attribution) license
- Mucosa-associated Lymphoid Tissue (MALT) Nodule © OpenStax A&P is licensed under a CC BY (Attribution) license
- Innate Immune System Table © OpenStax Concepts of Biology is licensed under a CC BY (Attribution) license
- Phagocytic Cells © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Granular Leukocytes © OpenStax Anatomy and Physiology is licensed under a CC BY (Attribution) license
- An APC (Antigen Presenting Cell) © OpenStax Ap Bio is licensed under a CC BY (Attribution) license
- Natural Killer (NK) Cells © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Innate Immune System Cells Chart © OpenStax Bio 2e is licensed under a CC BY (Attribution) license
- Acute Inflammation © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Interferons © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- The Classic Pathway for the Complement Cascade © OpenStax is licensed under a CC BY (Attribution) license
- Ahi Poke © Sanba38 is licensed under a CC BY-SA (Attribution ShareAlike) license
- Extravasation (Diapedesis) of Leukocytes © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Antigens © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Major Histocompatibility Complex (MHC) © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Naïve CD4+ T Cells © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- Helper T Cell © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- T-independent Antigens © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- B Cell Activation © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- B-Cell Receptor © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Table of Antibody Isotypes © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Secondary Antibody Response © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Antibodies © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Antibodies Serve as Opsonins © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Neutralization © OS Microbiology is licensed under a CC BY (Attribution) license
- Generating Lymphocyte Receptor Variability © OpenStax AP Bio is licensed under a CC BY (Attribution) license
- Differentiation of T Cells within the Thymus © OpenStax Anatomy and Physiology is licensed under a CC BY (Attribution) license
- Clonal Selection of B Cells © OpenStax Anatomy and Physiology is licensed under a CC BY (Attribution) license
- Primary and Secondary Immune Adaptive Response © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- The Four Classifications of Immunity © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Angiostrongylus Cantonensi: The life cycle of angiostrongyliasis © Wang, Q. P., Lai, D. H., Zhu, X. Q., Chen, X. G., & Lun, Z. R is licensed under a CC BY-SA (Attribution ShareAlike) license
- Angiostrongylus Cantonensi: Adult female worm of Angiostrongylus Cantonensis © John F. Lindo, Cecilia Waugh, John Hall, Colette Cunningham-Myrie, Deanna Ashley, Mark L. Eberhard, James J. Sullivan, Henry S. Bishop, David G. Robinson, Timothy Holtz, and Ralph D. Robinson is licensed under a Public Domain license
- HIV Symptoms and Replication: Early symptoms of HIV © Nicolearyanne is licensed under a CC BY-SA (Attribution ShareAlike) license
- HIV Symptoms and Replication: The HIV replication cycle © Jmarchn is licensed under a CC BY-SA (Attribution ShareAlike) license
- HIV Disease Progression © OpenStax Anatomy and Physiology is licensed under a CC BY (Attribution) license
- Coronavirus © NIH Image Gallery is licensed under a Public Domain license
- Immunological Hypersensitivity © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Skin Prick Test © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Type III Hypersensitivities and the Systems They Affect © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Type IV Hypersensitivity © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Types of Hypersensitivities © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Goiter © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Exophthalmia © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Rheumatoid Arthritis © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Lupus © OpenStax Microbiology is licensed under a CC BY (Attribution) license
- Honu_‘Iwalani Clayton_CCBY_2022 10 30 © ‘Iwalani Clayton is licensed under a CC BY (Attribution) license
- divider_maile
abnormal functioning part of the body, illness, sickness or ailment
a pathology-inducing agent such as certain bacteria, fungi or viruses
single-celled, prokaryotic microorganisms
a disease caused by a Mycobacterium leprae infection primarily affecting the peripheral nervous system, skin, and nasal mucosa
an abnormal sensation, such as prickling, itching, etc.
the extent or ability of a pathogen to cause disease
a non-living infectious agent that requires a living cell to multiply
eukaryotic organisms distinguished from animals and plants by chitin-containing cell walls among other features
a protein folded into an abnormal shape that causes normal proteins to also misfold
(aka normal flora) the typical bacteria, viruses and other microbes associated with healthy populations
(also, white blood cell) colorless, nucleated blood cell, the chief function of which is to protect the body from disease
a relatively short chain of amino acids with anti-bacterial, -viral or -fungal properties
soluble, short-range, cell-to-cell communication molecule
network of lymphatic vessels, lymph nodes, and ducts that carries lymph from the tissues and back to the bloodstream
fluid between cells and tissues, excluding plasma or lymph
smallest of the lymphatic vessels and the origin of lymph flow
fluid contained within the lymphatic system
projecting, hair-like structures that prevent lymphatic capillary collapse
openings that allow IF to enter lymphatic capillaries, becoming lymph
increase in the volume of the thorax during inhalation that decreases air pressure, enabling venous blood to flow into the thoracic region, then exhalation increases pressure, moving blood into the atria
inward projections within lymphatic capillaries that prevent lymph backflow
Lymphatic vasculature that form from fused smaller lymphatic capillaries, these deliver lymph to and from lymph nodes
one of the bean-shaped organs found associated with the lymphatic vessels
lead into a lymph node
lead out of a lymph node
large lymphatics that collect lymph from smaller lymphatic vessels and empties into the
blood via lymphatic ducts
drains lymph fluid from the upper right side of body into the right subclavian
vein
large duct that drains lymph from the lower limbs, left thorax, left upper limb, and the left
side of the head
organ that is involved in the development and maturation of T-cells and is particularly active during infancy and childhood
site where lymphocytes mature and proliferate; red bone marrow and
thymus gland
sites where lymphocytes mount adaptive immune responses; examples include lymph nodes and spleen
areas within the spleen where old, damaged RBCs are broken down
areas in the spleen where numerous WBCs are performing immunological functions
agranular leukocytes of the lymphoid stem cell line, many of which function in specific immunity
molecule recognized by the receptors of B and T lymphocytes
lymphoid nodules associated with the nasopharynx
lymphoid follicles associated with distal regions of the small intestine, part of the MALT
mucosa-associated lymphoid tissue, lymphoid tissue associated with the digestive tract and respiratory system
bronchus-associated lymphoid tissue, lymphoid nodule associated with the respiratory tract
lymphatic capillaries of the gut that absorb bile to form chyle
lipid-rich lymph inside the lymphatic capillaries of the small intestine
bag-like vessel that forms the beginning of the thoracic duct
rapid but relatively nonspecific immune responses
leukocyte receptor that binds to specific cell wall components of different bacterial species
perforin molecule in NK cell and cytotoxic T cell granules that form pores in the membrane of a target cell
pathogen associated molecular patterns that are recognized by PRRs of the innate immune system
Endocytosis of large particles.
damage-associated molecular patterns recognized by PRRs of innate immunity
receptors on cells of the innate immune system that bind PAMPs and DAMPs
WBCs that have granules; neutrophils, eosinophils and basophils
WBCs that aren’t granulocytes including monocytes, dendritic cells and lymphocytes
phagocytic white blood cell recruited from the bloodstream to the site of infection via the
bloodstream
granulocytes specialised for attaching parasites
granulocytes with histidine containing granules and are implicated in allergies
an antigen presenting cells, namely macrophages and dendritic cells
phagocytic cell of the myeloid lineage; a matured monocyte
polymorphonuclear cells, aka granulocytes
neutrophil extracellular traps, released DNA that traps and thereby sequestering pathogens
cell found in the skin and the lining of body cells that contains cytoplasmic granules with
vasoactive mediators such as histamine (similar to basophils)
Chemical compound released by mast cells in response to injury that causes vasodilation and endothelium permeability.
cytotoxic lymphocyte of innate immune response
precursor to macrophages and dendritic cells seen in the blood
an APC similar to macrophages
a cell capable of phagocytosis, namely neutrophils, macrophages and dendritic cells
binding of processed antigen to the protein-binding cleft of a major histocompatibility complex molecule
an enzyme that forms holes in the plasma membrane, released by NK and Tc cells
apoptosis-inducing substance contained in granules of NK cells and cytotoxic T cells
Response of tissue to injury.
signaling molecules that may function in hemopoiesis, inflammation, and specific immune responses
colony-stimulating factors, induce proliferation of WBCs in the bone marrow
soluble, long-range, cell-to-cell communication molecule
leukocyte proliferation in bone marrow
early induced proteins made in virally infected cells that cause nearby cells to make antiviral proteins
abnormally high body temperature, aka pyrexia
peptides that contribute to pain, inflammation and smooth muscle contraction
lipids associated with inflammation, bronchodilation and many other effects
enzymatic cascade of constitutive blood proteins that have anti-pathogen effects, including
the direct killing of bacteria
Widening of blood vessels.
increased blood flow near the site of inflammation
antigen-specific protein secreted by plasma cells; immunoglobulin
complement protein that fragments into C3a and C3b. C3a enhances inflammation and C3b is an opsonin and activates C5
complement activated by the presence of certain carbohydrates typical of some bacteria
complement proteins are activated by the presence of pathogen bound antibodies
protein antibody; occurs as one of five main classes
complement protein that fragments into C5a and C5b. C5a enhances inflammation C5b initiates formation of the MAC by triggering the interaction of C6, C7, C8 and several C9s
membrane attack complex, the interaction of C5, C6, C7, C8 and several C9s creates a hole in bacterial membranes disrupting the targeted cell
non-steroidal anti-inflammatory drugs such as aspirin and ibuprofen
abnormally high body temperature, aka fever
chemicals that signal the hypothalamus to increase body temperature
fever-reducing medications
a chemical class type of AMPs
a chemical class type of AMPs
cell adhesion molecules that promote intercellular binding
excessive leukocyte proliferation
instead of the middle of the blood vessel, cells travel along the wall by sticking and rolling via CAM interactions
(also, emigration) process by which leukocytes squeeze through adjacent cells in a blood vessel wall to enter tissues
cells following a chemical concentration gradient such as leukocytes following a chemokine trail toward a site of injury
relatively slow but very specific and effective immune response involving lymphocytes
the specific region of an antigen that binds to a T or B cell receptor
lymphocyte that acts by differentiating into an antibody-secreting plasma cell
antibodies blocking disease promoting molecules on a pathogens such as adherence proteins
ability of the adaptive immune response to mount a stronger and faster immune response upon re-exposure to a pathogen
gene cluster whose proteins present antigens to T cells
found on most cells of the body, it binds to the CD8 molecule on T cells
T cells that secrete cytokines to enhance other immune responses, involved in
activation of both B and Tc cell lymphocytes
multiple MHC genes and their proteins found in body cells
multiple alleles for each individual MHC locus
Th , helper T cells, T cells that express the surface protein CD4
immune cells with a direct, adverse effect on a pathogen
plasma cells, activated B lymphocytes
class of CD4 T cells that regulates other T cell responses
adaptive immunity activated by helper T cell subclass Th1, involving TC
cells that secrete cytokines that enhance the activity of Tc
T lymphocytes with the ability to induce apoptosis in target cells
molecule expressed on cytotoxic T cells and NK cells that binds to the fas molecule on a
target cell and induces it do undergo apoptosis
differentiated B cell that is actively secreting antibody
cells that secrete cytokines that induce B cells to differentiate into plasma cells
interleukin-4, promotes Th2 and plasma cell development among other functions
adaptive immunity involving Th2 stimulating B cells to secrete antibodies
binds to B cells, which do not require signals from T cells to make antibody
antigen that binds to B cells, which requires signals from T cells to make antibody
fragment of antigen binding, that part of the antibody that binds antigen
domain part of a lymphocyte antigen receptor that varies considerably between different
receptor types
in an antibody molecule, the site where the two termini of the heavy chains come together;
many cells have receptors for this portion of the antibody, adding functionality to these molecules
domain part of a lymphocyte antigen receptor that does not vary much between different
receptor types
main blood antibody of late primary and early secondary responses; passed from carrier to unborn child via placenta
class of antibody whose only known function is as a receptor on naive B cells; important in B cell activation
antibody whose monomer is a surface receptor of naive B cells; the pentamer is the first antibody made blood plasma during primary responses
ability of B cells to change the class of antibody they produce without altering the
specificity for antigen, aka isotype switching
antibody whose dimer is secreted by exocrine glands, is especially effective against digestive and respiratory pathogens, and can pass immunity to an infant through breastfeeding
antibody that binds to mast cells and causes antigen-specific degranulation during an allergic response
a membrane bound protein on phagocytes that binds the Fc region of an antibody
clustering of cells into masses linked by antibodies
enhancement of phagocytosis by the binding of antibody or antimicrobial protein
the genes responsible for recombining segments and randomly inserting nucleotides into the variable regions of T and B cell receptor genes
selection of thymocytes within the thymus that interact with self, but not non-self, MHC molecules
selection against thymocytes in the thymus that react with self-antigen neutralization inactivation of a virus by the binding of specific antibody
molecules produced by and that do not induce adaptive immune responses of an individual in the absence of autoimmune disease
B cell tolerance induced in immature B cells of the bone marrow
a pathologic adaptive immune response against your own tissues
mature B or T cell that has not yet encountered antigen for the first time
vessels containing unique endothelial cells specialized to allow migration of
lymphocytes from the blood to the lymph node
proliferation of B lymphocytes with a specific antigen receptor into a population with varying degree of antigen binding strength
clusters of rapidly proliferating B cells found in secondary lymphoid tissues
the process of the antigen-binding regions of the B cell receptor (and antibody) to be altered during B cell clonal expansion
long-lived T or B cell reserved for future exposure to a pathogen
immune system’s response to the first exposure to a pathogen
immune response observed upon re-exposure to a pathogen, which is
stronger and faster than a primary response
immunity developed from an individual’s own immune system
the normal exposure to a pathogen or toxin causing an immune response
occurs when an antigen is intentionally injected into the body for the sake of generating antibodies and memory lymphocytes such as with vaccines
transfer of immunity to a pathogen to an individual that lacks immunity to this pathogen
usually by the injection of antibodies
the degree to which an antigen binds a T or B cell receptor
the degree by which an antigen induces an immune response
a disease capable of transmitting from person to person
endemic
the rapid spreading or temporary prevalence of disease in a population
an epidemic of a large area such as an entire country, continent, or planet
rat lungworm disease, caused by the parasitic nematode Angiostrongylus cantonensis carried by slugs and snails
is a virus capable of causing acquired immunodeficiency syndrome (AIDS), a serious and lethal disease characterized by a greatly weakened immune system
a serious and lethal disease caused by human immunodeficiency virus (HIV) and characterized by a greatly weakened immune system
clearance of pathogen in the serum and the simultaneous rise of serum antibody
pathogens that don’t normally cause disease in healthy individuals but can when the immune system is compromised in some way
coronavirus disease-2019 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for a worldwide pandemic in the year 2020
forecasting the probable outcome of disease including chances of recovery
an excessive, potentially harmful overreaction to an antigen
immediate response mediated by mast cell degranulation caused by the crosslinking of the antigen-specific IgE molecules on the mast cell surface
IgE-mediated mast cell degranulation caused by crosslinking of surface IgE by antigen
immune system series of barriers, cells, and soluble mediators that combine to response to infections of the body with pathogenic organisms
antigens responsible for type I hypersensitivity
a drug that counteracts the effects of histamine
damage to tissues caused by the deposition of antibody-antigen (immune) complexes followed by the activation of complement
cell damage caused by the binding of antibody and the activation of complement, usually against red blood cells
(aka delayed hypersensitivity)
an antibody-independent response primarily involving by T cells and macrophages
T cell-mediated immune response against pathogens infiltrating interstitial tissues, causing cellular infiltrate
is an autoimmune disease that causes inflammation, pain, stiffness, and loss of function of the joints
an autoimmune disease causing inflammation, joint aches, fever, and characteristic skin rashes and swelling
an autoimmune disease causing hyperthyroidism resulting in tachycardia, heat sensitivity, weight loss, eye bulging among other symptoms
an infection with Streptococcus resulting in inflammation heart, skin, joints or brain tissue
study of the connections between the immune, nervous, and endocrine
systems
diseases whose symptoms are brought about or worsened by stress and emotional factors
a protein associated with stress, inflammation and psychophysiological disorders

